Temporal and sex differences in the role of BDNF/TrkB signaling in hyperalgesic priming in mice and rats
- PMID: 31194015
- PMCID: PMC6550116
- DOI: 10.1016/j.ynpai.2018.10.001
Temporal and sex differences in the role of BDNF/TrkB signaling in hyperalgesic priming in mice and rats
Abstract
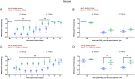
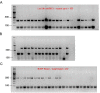
Brain-derived neurotrophic factor (BDNF) signaling through its cognate receptor, TrkB, is a well-known promoter of synaptic plasticity at nociceptive synapses in the dorsal horn of the spinal cord. Existing evidence suggests that BDNF/TrkB signaling in neuropathic pain is sex dependent. We tested the hypothesis that the effects of BDNF/TrkB signaling in hyperalgesic priming might also be sexually dimorphic. Using the incision postsurgical pain model in male mice, we show that BDNF sequestration with TrkB-Fc administered at the time of surgery blocks the initiation and maintenance of hyperalgesic priming. However, when BDNF signaling was blocked prior to the precipitation of hyperalgesic priming with prostaglandin E2 (PGE2), priming was not reversed. This result is in contrast to our findings in male mice with interleukin-6 (IL6) as the priming stimulus where TrkB-Fc was effective in reversing the maintenance of hyperalgesic priming. Furthermore, in IL6-induced hyperalgesic priming, the BDNF sequestering agent, TrkB-fc, was effective in reversing the maintenance of hyperalgesic priming in male mice; however, when this experiment was conducted in female mice, we did not observe any effect of TrkB-fc. This markedly sexual dimorphic effect in mice is consistent with recent studies showing a similar effect in neuropathic pain models. We tested whether the sexual dimorphic role for BDNF was consistent across species. Importantly, we find that this sexual dimorphism does not occur in rats where TrkB-fc reverses hyperalgesic priming fully in both sexes. Finally, to determine the source of BDNF in hyperalgesic priming in mice, we used transgenic mice (Cx3cr1CreER × Bdnfflx/flx mice) with BDNF eliminated from microglia. From these experiments we conclude that BDNF from microglia does not contribute to hyperalgesic priming and that the key source of BDNF for hyperalgesic priming is likely nociceptors in the dorsal root ganglion. These experiments demonstrate the importance of testing mechanistic hypotheses in both sexes in multiple species to gain insight into complex biology underlying chronic pain.
Keywords: BDNF; Hyperalgesic priming; IL6; Sex differences; TrkB.
Figures



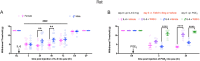

Similar articles
-
Spinal brain-derived neurotrophic factor (BDNF) produces hyperalgesia in normal mice while antisense directed against either BDNF or trkB, prevent inflammation-induced hyperalgesia.Pain. 2002 Nov;100(1-2):171-81. doi: 10.1016/s0304-3959(02)00264-6. Pain. 2002. PMID: 12435470
-
Spinal Inhibition of P2XR or p38 Signaling Disrupts Hyperalgesic Priming in Male, but not Female, Mice.Neuroscience. 2018 Aug 10;385:133-142. doi: 10.1016/j.neuroscience.2018.06.012. Epub 2018 Jun 18. Neuroscience. 2018. PMID: 29913243 Free PMC article.
-
Protease-activated receptor 2 activation is sufficient to induce the transition to a chronic pain state.Pain. 2015 May;156(5):859-867. doi: 10.1097/j.pain.0000000000000125. Pain. 2015. PMID: 25734998 Free PMC article.
-
Functional recovery after cervical spinal cord injury: Role of neurotrophin and glutamatergic signaling in phrenic motoneurons.Respir Physiol Neurobiol. 2016 Jun;226:128-36. doi: 10.1016/j.resp.2015.10.009. Epub 2015 Oct 23. Respir Physiol Neurobiol. 2016. PMID: 26506253 Free PMC article. Review.
-
Hyperalgesic Priming in the Transition From Acute to Chronic Pain: Focus on Different Models and the Molecular Mechanisms Involved.J Pain Res. 2025 Mar 21;18:1491-1501. doi: 10.2147/JPR.S514851. eCollection 2025. J Pain Res. 2025. PMID: 40135188 Free PMC article. Review.
Cited by
-
Synapse Regulation.Adv Neurobiol. 2024;37:179-208. doi: 10.1007/978-3-031-55529-9_11. Adv Neurobiol. 2024. PMID: 39207693 Review.
-
Neurofibromatosis type 1-dependent alterations in mouse microglia function are not cell-intrinsic.Acta Neuropathol Commun. 2023 Mar 9;11(1):36. doi: 10.1186/s40478-023-01525-w. Acta Neuropathol Commun. 2023. PMID: 36890585 Free PMC article.
-
Brain-derived neurotrophic factor contributes to activity-induced muscle pain in male but not female mice.Brain Behav Immun. 2024 Aug;120:471-487. doi: 10.1016/j.bbi.2024.06.019. Epub 2024 Jun 24. Brain Behav Immun. 2024. PMID: 38925417 Free PMC article.
-
Sex differences in pain along the neuraxis.Neuropharmacology. 2022 Jun 1;210:109030. doi: 10.1016/j.neuropharm.2022.109030. Epub 2022 Mar 21. Neuropharmacology. 2022. PMID: 35331712 Free PMC article. Review.
-
The Role of Astrocytes in the Modulation ofK+-Cl--Cotransporter-2 Function.Int J Mol Sci. 2020 Dec 15;21(24):9539. doi: 10.3390/ijms21249539. Int J Mol Sci. 2020. PMID: 33333849 Free PMC article. Review.
References
-
- Banik R.K., Woo Y.C., Park S.S., Brennan T.J. Strain and sex influence on pain sensitivity after plantar incision in the mouse. Anesthesiology. 2006;105:1246–1253. - PubMed
-
- Buvanendran A., Kroin J.S., Berger R.A., Hallab N.J., Saha C., Negrescu C., Moric M., Caicedo M.S., Tuman K.J. Upregulation of prostaglandin E2 and interleukins in the central nervous system and peripheral tissue during and after surgery in humans. Anesthesiology. 2006;104:403–410. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

