Human-like cutaneous neuropathologies associated with a porcine model of peripheral neuritis: A translational platform for neuropathic pain
- PMID: 31194066
- PMCID: PMC6550106
- DOI: 10.1016/j.ynpai.2018.07.002
Human-like cutaneous neuropathologies associated with a porcine model of peripheral neuritis: A translational platform for neuropathic pain
Abstract
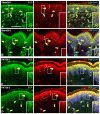
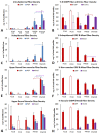
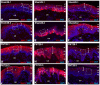
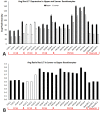
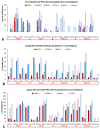
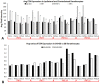
Despite enormous investment in research and development of novel treatments, there remains a lack of predictable, effective, and safe therapeutics for human chronic neuropathic pain (NP) afflictions. NP continues to increase among the population and treatments remain a major unmet public health care need. In recent years, numerous costly (time and money) failures have occurred attempting to translate successful animal pain model results, typically using rodents, to human clinical trials. These continued failures point to the essential need for better animal models of human pain conditions. To address this challenge, we have previously developed a peripheral neuritis trauma (PNT) model of chronic pain induced by a proximal sciatic nerve irritation in pigs, which have a body size, metabolism, skin structure, and cutaneous innervation more similar to humans. Here, we set out to determine the extent that the PNT model presents with cutaneous neuropathologies consistent with those associated with human chronic NP afflictions. Exactly as is performed in human skin biopsies, extensive quantitative multi-molecular immunofluorescence analyses of porcine skin biopsies were performed to assess cutaneous innervation and skin structure. ChemoMorphometric Analysis (CMA) results demonstrated a significant reduction in small caliber intraepidermal nerve fiber (IENF) innervation, altered dermal vascular innervation, and aberrant analgesic/algesic neurochemical properties among epidermal keratinocytes, which are implicated in modulating sensory innervation. These comprehensive pathologic changes very closely resemble those observed from CMA of human skin biopsies collected from NP afflictions. The results indicate that the porcine PNT model is more appropriate for translational NP research compared with commonly utilized rodent models. Because the PNT model creates cutaneous innervation and keratinocyte immunolabeling alterations consistent with human NP conditions, use of this animal model for NP testing and treatment response characteristics will likely provide more realistic results to direct successful translation to humans.
Keywords: Animal model; CGRP; ET-1 receptors; IENF density; Immunolabeling; NaV; PGP9.5.
Figures














Similar articles
-
Keratinocyte Biomarkers Distinguish Painful Diabetic Peripheral Neuropathy Patients and Correlate With Topical Lidocaine Responsiveness.Front Pain Res (Lausanne). 2021 Dec 8;2:790524. doi: 10.3389/fpain.2021.790524. eCollection 2021. Front Pain Res (Lausanne). 2021. PMID: 35295428 Free PMC article.
-
Pain-related changes in cutaneous innervation of patients suffering from bortezomib-induced, diabetic or chronic idiopathic axonal polyneuropathy.Brain Res. 2020 Mar 1;1730:146621. doi: 10.1016/j.brainres.2019.146621. Epub 2020 Jan 9. Brain Res. 2020. PMID: 31926911
-
Peripheral Neuritis Trauma in Pigs: A Neuropathic Pain Model.J Pain. 2016 Jan;17(1):36-49. doi: 10.1016/j.jpain.2015.09.011. Epub 2015 Oct 9. J Pain. 2016. PMID: 26456763
-
European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society.J Peripher Nerv Syst. 2010 Jun;15(2):79-92. doi: 10.1111/j.1529-8027.2010.00269.x. J Peripher Nerv Syst. 2010. PMID: 20626771 Review.
-
A mechanistic understanding of the relationship between skin innervation and chemotherapy-induced neuropathic pain.Front Pain Res (Lausanne). 2022 Dec 13;3:1066069. doi: 10.3389/fpain.2022.1066069. eCollection 2022. Front Pain Res (Lausanne). 2022. PMID: 36582196 Free PMC article. Review.
Cited by
-
Cutaneous innervation in impaired diabetic wound healing.Transl Res. 2021 Oct;236:87-108. doi: 10.1016/j.trsl.2021.05.003. Epub 2021 May 23. Transl Res. 2021. PMID: 34029747 Free PMC article. Review.
-
Non-invasive central nervous system assessment of a porcine model of neuropathic pain demonstrates increased latency of somatosensory-evoked potentials.J Neurosci Methods. 2023 Aug 1;396:109934. doi: 10.1016/j.jneumeth.2023.109934. Epub 2023 Jul 29. J Neurosci Methods. 2023. PMID: 37524248 Free PMC article.
-
Cutaneous targets for topical pain medications in patients with neuropathic pain: individual differential expression of biomarkers supports the need for personalized medicine.Pain Rep. 2024 Feb 16;9(2):e1119. doi: 10.1097/PR9.0000000000001121. eCollection 2024 Apr. Pain Rep. 2024. PMID: 38375092 Free PMC article.
-
Keratinocyte Biomarkers Distinguish Painful Diabetic Peripheral Neuropathy Patients and Correlate With Topical Lidocaine Responsiveness.Front Pain Res (Lausanne). 2021 Dec 8;2:790524. doi: 10.3389/fpain.2021.790524. eCollection 2021. Front Pain Res (Lausanne). 2021. PMID: 35295428 Free PMC article.
-
Spared ulnar nerve injury results in increased layer III-VI excitability in the pig somatosensory cortex.Lab Anim (NY). 2024 Oct;53(10):287-293. doi: 10.1038/s41684-024-01440-0. Epub 2024 Sep 30. Lab Anim (NY). 2024. PMID: 39349800 Free PMC article.
References
-
- Albrecht P.J., Hines S., Eisenberg E., Pud D., Finlay D.R., Connolly M.K., Pare M., Davar G., Rice F.L. Pathologic alterations of cutaneous innervation and vasculature in affected limbs from patients with complex regional pain syndrome. Pain. 2006;120(3):244–266. - PubMed
-
- Albrecht P.J., Hou Q., Argoff C.E., Storey J.R., Wymer J.P., Rice F.L. Excessive peptidergic sensory innervation of cutaneous arteriole-venule shunts (Avs) in the palmar glabrous skin of fibromyalgia patients: implications for widespread deep tissue pain and fatigue. Pain Med. 2013;14(6):895–915. - PubMed
-
- Albrecht P.J., Rice F.L. Role of small-fiber afferents in pain mechanisms with implications on diagnosis and treatment. Curr. Pain Headache Rep. 2010;14(3):179–188. - PubMed
-
- Bagnato A., Venuti A., Di Castro V., Marcante M.L. Identification of the eta receptor subtype that mediates endothelin induced autocrine proliferation of normal human keratinocytes. Biochem. Biophys. Res. Commun. 1995;209(1):80–86. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

