Open-label Clinical Trial of Niraparib Combined With Pembrolizumab for Treatment of Advanced or Metastatic Triple-Negative Breast Cancer
- PMID: 31194225
- PMCID: PMC6567845
- DOI: 10.1001/jamaoncol.2019.1029
Open-label Clinical Trial of Niraparib Combined With Pembrolizumab for Treatment of Advanced or Metastatic Triple-Negative Breast Cancer
Abstract
Importance: Poly(adenosine diphosphate-ribose) polymerase inhibitor and anti-programmed death receptor-1 inhibitor monotherapy have shown limited clinical activity in patients with advanced triple-negative breast cancer (TNBC).
Objective: To evaluate the clinical activity (primary) and safety (secondary) of combination treatment with niraparib and pembrolizumab in patients with advanced or metastatic TNBC.
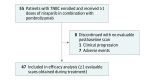
Design, setting, and participants: This open-label, single-arm, phase 2 study enrolled 55 eligible patients with advanced or metastatic TNBC irrespective of BRCA mutation status or programmed death-ligand 1 (PD-L1) expression at 34 US sites. Data were collected from January 3, 2017, through October 29, 2018, and analyzed from October 29, 2018, through February 27, 2019.
Interventions: Patients were administered 200 mg of oral niraparib once daily in combination with 200 mg of intravenous pembrolizumab on day 1 of each 21-day cycle.
Main outcomes and measures: The primary end point was objective response rate (ORR) per the Response Evaluation Criteria in Solid Tumors, version 1.1. Secondary end points were safety, disease control rate (DCR; complete response plus partial response plus stable disease), duration of response (DOR), progression-free survival (PFS), and overall survival.
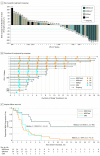
Results: Within the full study population of 55 women (median age, 54 years [range, 32-90 years]), 5 patients had confirmed complete responses, 5 had confirmed partial responses, 13 had stable disease, and 24 had progressive disease. In the efficacy-evaluable population (n = 47), ORR included 10 patients (21%; 90% CI, 12%-33%) and DCR included 23 (49%; 90% CI, 36%-62%). Median DOR was not reached at the time of the data cutoff, with 7 patients still receiving treatment at the time of analysis. In 15 evaluable patients with tumor BRCA mutations, ORR included 7 patients(47%; 90% CI, 24%-70%), DCR included 12 (80%; 90% CI, 56%-94%), and median PFS was 8.3 months (95% CI, 2.1 months to not estimable). In 27 evaluable patients with BRCA wild-type tumors, ORR included 3 patients (11%; 90% CI, 3%-26%), DCR included 9 (33%; 90% CI, 19%-51%), and median PFS was 2.1 months (95% CI, 1.4-2.5 months). The most common treatment-related adverse events of grade 3 or higher were anemia (10 [18%]), thrombocytopenia (8 [15%]), and fatigue (4 [7%]). Immune-related adverse events were reported in 8 patients (15%) and were grade 3 in 2 patients (4%); no new safety signals were detected.
Conclusions and relevance: Combination niraparib plus pembrolizumab provides promising antitumor activity in patients with advanced or metastatic TNBC, with numerically higher response rates in those with tumor BRCA mutations. The combination therapy was safe with a tolerable safety profile, warranting further investigation.
Trial registration: ClinicalTrials.gov identifier: NCT02657889.
Conflict of interest statement
Figures
Comment in
-
BRCA Mutations and Homologous Recombination Repair Deficiency in Treatment With Niraparib Combined With Pembrolizumab.JAMA Oncol. 2020 Mar 1;6(3):440-441. doi: 10.1001/jamaoncol.2019.4595. JAMA Oncol. 2020. PMID: 31876918 No abstract available.
References
-
- National Comprehensive Cancer Network Clinical practice guidelines in oncology: breast cancer version 1.2018. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed July 2, 2018.
-
- Gradishar WJ, Kaklamani V, Sahoo TP, et al. . A double-blind, randomised, placebo-controlled, phase 2b study evaluating sorafenib in combination with paclitaxel as a first-line therapy in patients with HER2-negative advanced breast cancer. Eur J Cancer. 2013;49(2):312-322. doi:10.1016/j.ejca.2012.08.005 - DOI - PubMed
-
- Kim SB, Dent R, Im SA, et al. ; LOTUS Investigators . Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2017;18(10):1360-1372. doi:10.1016/S1470-2045(17)30450-3 - DOI - PMC - PubMed
-
- Miles D, Cameron D, Bondarenko I, et al. . Bevacizumab plus paclitaxel versus placebo plus paclitaxel as first-line therapy for HER2-negative metastatic breast cancer (MERIDIAN): a double-blind placebo-controlled randomised phase III trial with prospective biomarker evaluation. Eur J Cancer. 2017;70:146-155. doi:10.1016/j.ejca.2016.09.024 - DOI - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

