PeIA-5466: A Novel Peptide Antagonist Containing Non-natural Amino Acids That Selectively Targets α3β2 Nicotinic Acetylcholine Receptors
- PMID: 31194549
- PMCID: PMC7342494
- DOI: 10.1021/acs.jmedchem.9b00566
PeIA-5466: A Novel Peptide Antagonist Containing Non-natural Amino Acids That Selectively Targets α3β2 Nicotinic Acetylcholine Receptors
Abstract
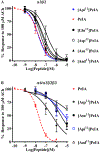
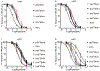
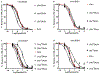
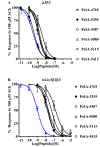
Pharmacologically distinguishing α3β2 nicotinic acetylcholine receptors (nAChRs) from closely related subtypes, particularly α6β2, has been challenging due to the lack of subtype-selective ligands. We created analogs of α-conotoxin (α-Ctx) PeIA to identify ligand-receptor interactions that could be exploited to selectively increase potency and selectivity for α3β2 nAChRs. A series of PeIA analogs were synthesized by replacing amino acid residues in the second disulfide loop with standard or nonstandard residues and assessing their activity on α3β2 and α6/α3β2β3 nAChRs heterologously expressed in Xenopus laevis oocytes. Asparagine11 was found to occupy a pivotal position, and when replaced with negatively charged amino acids, selectivity for α3β2 over α6/α3β2β3 nAChRs was substantially increased. Second generation peptides were then designed to further improve both potency and selectivity. One peptide, PeIA-5466, was ∼300-fold more potent on α3β2 than α6/α3β2β3 and is the most α3β2-selective antagonist heretofore reported.
Conflict of interest statement
The authors declare no competing financial interest
Figures

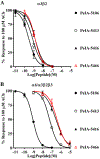

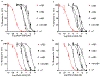
Similar articles
-
Positional scanning mutagenesis of α-conotoxin PeIA identifies critical residues that confer potency and selectivity for α6/α3β2β3 and α3β2 nicotinic acetylcholine receptors.J Biol Chem. 2013 Aug 30;288(35):25428-25439. doi: 10.1074/jbc.M113.482059. Epub 2013 Jul 11. J Biol Chem. 2013. PMID: 23846688 Free PMC article.
-
A novel α4/7-conotoxin LvIA from Conus lividus that selectively blocks α3β2 vs. α6/α3β2β3 nicotinic acetylcholine receptors.FASEB J. 2014 Apr;28(4):1842-53. doi: 10.1096/fj.13-244103. Epub 2014 Jan 7. FASEB J. 2014. PMID: 24398291 Free PMC article.
-
α-Conotoxin PeIA[S9H,V10A,E14N] potently and selectively blocks α6β2β3 versus α6β4 nicotinic acetylcholine receptors.Mol Pharmacol. 2012 Nov;82(5):972-82. doi: 10.1124/mol.112.080853. Epub 2012 Aug 22. Mol Pharmacol. 2012. PMID: 22914547 Free PMC article.
-
α-Conotoxin Bt1.8 from Conus betulinus selectively inhibits α6/α3β2β3 and α3β2 nicotinic acetylcholine receptor subtypes.J Neurochem. 2021 Oct;159(1):90-100. doi: 10.1111/jnc.15434. Epub 2021 Jun 22. J Neurochem. 2021. PMID: 34008858
-
The Structural Features of α-Conotoxin Specifically Target Different Isoforms of Nicotinic Acetylcholine Receptors.Curr Top Med Chem. 2015;16(2):156-69. doi: 10.2174/1568026615666150701114831. Curr Top Med Chem. 2015. PMID: 26126912 Review.
Cited by
-
Analogs of α-conotoxin PnIC selectively inhibit α7β2- over α7-only subtype nicotinic acetylcholine receptors via a novel allosteric mechanism.FASEB J. 2024 Jan;38(1):e23374. doi: 10.1096/fj.202302079. FASEB J. 2024. PMID: 38161283 Free PMC article.
-
Computational Design of α-Conotoxins to Target Specific Nicotinic Acetylcholine Receptor Subtypes.Chemistry. 2024 Feb 1;30(7):e202302909. doi: 10.1002/chem.202302909. Epub 2023 Dec 18. Chemistry. 2024. PMID: 37910861 Free PMC article.
-
Molecular Determinants of Species Specificity of α-Conotoxin TxIB towards Rat and Human α6/α3β4 Nicotinic Acetylcholine Receptors.Int J Mol Sci. 2023 May 11;24(10):8618. doi: 10.3390/ijms24108618. Int J Mol Sci. 2023. PMID: 37239959 Free PMC article.
-
Single Amino Acid Substitution in Loop1 Switches the Selectivity of α-Conotoxin RegIIA towards the α7 Nicotinic Acetylcholine Receptor.Mar Drugs. 2024 Aug 29;22(9):390. doi: 10.3390/md22090390. Mar Drugs. 2024. PMID: 39330271 Free PMC article.
-
Alkaloid ligands enable function of homomeric human α10 nicotinic acetylcholine receptors.Front Pharmacol. 2022 Sep 16;13:981760. doi: 10.3389/fphar.2022.981760. eCollection 2022. Front Pharmacol. 2022. PMID: 36188578 Free PMC article.
References
-
- Gotti C; Zoli M; Clementi F Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol. Sci 2006, 27, 482–491. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

