CAR, a Homing Peptide, Prolongs Pulmonary Preferential Vasodilation by Increasing Pulmonary Retention and Reducing Systemic Absorption of Liposomal Fasudil
- PMID: 31194563
- PMCID: PMC7035787
- DOI: 10.1021/acs.molpharmaceut.9b00208
CAR, a Homing Peptide, Prolongs Pulmonary Preferential Vasodilation by Increasing Pulmonary Retention and Reducing Systemic Absorption of Liposomal Fasudil
Abstract
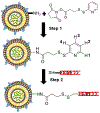
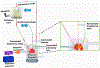
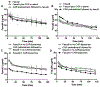
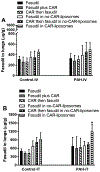
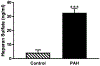
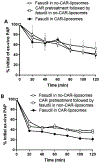
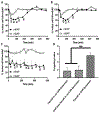
Here, we sought to elucidate the role of CAR (a cyclic peptide) in the accumulation and distribution of fasudil, a drug for pulmonary arterial hypertension (PAH), in rat lungs and in producing pulmonary specific vasodilation in PAH rats. As such, we prepared liposomes of fasudil and CAR-conjugated liposomal fasudil and assessed the liposomes for CAR conjugation, physical properties, entrapment efficiencies, in vitro release profiles, and stabilities upon incubation in cell culture media, storage, and aerosolization. We also studied the cellular uptake of fasudil in different formulations, quantified heparan sulfate (HS) in pulmonary arterial smooth muscle cells (PASMCs), and investigated the distribution of the liposomes in the lungs of PAH rats. We assessed the drug accumulation in a close and recirculating isolated perfused rat lung model and studied the pharmacokinetics and pharmacological efficacy of the drug and formulations in Sugen/hypoxia-induced PAH rats. The entrapment efficiency of the liposomal fasudil was 95.5 ± 4.5%, and the cumulative release was 93.95 ± 6.22%. The uptake of CAR liposomes by pulmonary arterial cells and their distribution and accumulation in the lungs were much greater than those of no-CAR-liposomes. CAR-induced increase in the cellular uptake was associated with an increase in HS expression by rat PAH-PASMCs. CAR, when conjugated with liposomal fasudil and given via an intratracheal instillation, extended the elimination half-life of the drug by four-fold compared with fasudil-in-no-CAR-liposomes given via the same route. CAR-conjugated liposomal fasudil, as opposed to fasudil-in-no-CAR-liposomes and CAR pretreatment followed by fasudil-in-no-CAR-liposomes, reduced the mean pulmonary arterial pressure by 40-50% for 6 h, without affecting the mean systemic arterial pressure. On the whole, this study suggests that CAR aids in concentrating the drug in the lungs, increasing the cellular uptake, extending the half-life of fasudil, and eliciting a pulmonary-specific vasodilation when the peptide remains conjugated on the liposomal surface, but not when CAR is given as a pretreatment or alone as an admixture with the drug.
Keywords: fasudil; isolated perfused rat lung; liposomes; peptide as a targeting moiety; pulmonary hypertension.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







Similar articles
-
Liposomal fasudil, a rho-kinase inhibitor, for prolonged pulmonary preferential vasodilation in pulmonary arterial hypertension.J Control Release. 2013 Apr 28;167(2):189-99. doi: 10.1016/j.jconrel.2013.01.011. Epub 2013 Jan 23. J Control Release. 2013. PMID: 23353807 Free PMC article.
-
Peptide-coated liposomal fasudil enhances site specific vasodilation in pulmonary arterial hypertension.Mol Pharm. 2014 Dec 1;11(12):4374-84. doi: 10.1021/mp500456k. Epub 2014 Nov 4. Mol Pharm. 2014. PMID: 25333706 Free PMC article.
-
Fasudil and DETA NONOate, Loaded in a Peptide-Modified Liposomal Carrier, Slow PAH Progression upon Pulmonary Delivery.Mol Pharm. 2018 May 7;15(5):1755-1765. doi: 10.1021/acs.molpharmaceut.7b01003. Epub 2018 Mar 26. Mol Pharm. 2018. PMID: 29528655 Free PMC article.
-
Evaluation of clinical efficacy of fasudil for the treatment of pulmonary arterial hypertension.Recent Pat Cardiovasc Drug Discov. 2012 Aug;7(2):100-4. doi: 10.2174/157489012801227238. Recent Pat Cardiovasc Drug Discov. 2012. PMID: 22670803 Review.
-
Safe and efficient drug delivery system with liposomes for intrathecal application of an antivasospastic drug, fasudil.Biol Pharm Bull. 2006 Mar;29(3):397-402. doi: 10.1248/bpb.29.397. Biol Pharm Bull. 2006. PMID: 16508135 Review.
Cited by
-
Current Overview of the Biology and Pharmacology in Sugen/Hypoxia-Induced Pulmonary Hypertension in Rats.J Aerosol Med Pulm Drug Deliv. 2024 Oct;37(5):241-283. doi: 10.1089/jamp.2024.0016. J Aerosol Med Pulm Drug Deliv. 2024. PMID: 39388691 Review.
-
Dual Affinity to RBCs and Target Cells (DART) Enhances Both Organ- and Cell Type-Targeting of Intravascular Nanocarriers.ACS Nano. 2022 Mar 22;16(3):4666-4683. doi: 10.1021/acsnano.1c11374. Epub 2022 Mar 10. ACS Nano. 2022. PMID: 35266686 Free PMC article.
-
Alleviating experimental pulmonary hypertension via co-delivering FoxO1 stimulus and apoptosis activator to hyperproliferating pulmonary arteries.Acta Pharm Sin B. 2023 Jun;13(6):2369-2382. doi: 10.1016/j.apsb.2022.12.002. Epub 2022 Dec 8. Acta Pharm Sin B. 2023. PMID: 37425053 Free PMC article.
-
Bioactive nanotherapeutic trends to combat triple negative breast cancer.Bioact Mater. 2021 Mar 13;6(10):3269-3287. doi: 10.1016/j.bioactmat.2021.02.037. eCollection 2021 Oct. Bioact Mater. 2021. PMID: 33778204 Free PMC article. Review.
-
Intratracheally Administered Peptide-Modified Lipid Admixture Containing Fasudil and/or DETA NONOate Ameliorates Various Pathologies of Pulmonary Arterial Hypertension.Pharmaceuticals (Basel). 2023 Nov 28;16(12):1656. doi: 10.3390/ph16121656. Pharmaceuticals (Basel). 2023. PMID: 38139783 Free PMC article.
References
-
- Iyer AK; Khaled G; Fang J; Maeda H Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discovery Today 2006, 11, 812–818. - PubMed
-
- Malam Y; Loizidou M; Seifalian AM Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer. Trends Pharmacol Sci. 2009, 30, 592–599. - PubMed
-
- Tacar O; Sriamornsak P; Dass C R Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol 2013, 65, 157–170. - PubMed
-
- Betancourt T; Byrne JD; Sunaryo N; Crowder SW; Kadapakkam M; Patel S; Casciato S; Brannon-Peppas L PEGylation strategies for active targeting of PLA/PLGA nanoparticles. J. Biomed. Mater. Res., Part A 2009, 91, 263–276. - PubMed
-
- Brooks NA; Pouniotis DS; Tang C-K; Apostolopoulos V; Pietersz GA Cell-penetrating peptides: application in vaccine delivery. Biochim. Biophys. Acta 2010, 1805, 25–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

