The use of high-frequency short bipolar pulses in cisplatin electrochemotherapy in vitro
- PMID: 31194692
- PMCID: PMC6572501
- DOI: 10.2478/raon-2019-0025
The use of high-frequency short bipolar pulses in cisplatin electrochemotherapy in vitro
Abstract
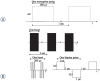
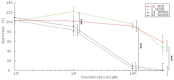
Background In electrochemotherapy (ECT), chemotherapeutics are first administered, followed by short 100 μs monopolar pulses. However, these pulses cause pain and muscle contractions. It is thus necessary to administer muscle relaxants, general anesthesia and synchronize pulses with the heart rhythm of the patient, which makes the treatment more complex. It was suggested in ablation with irreversible electroporation, that bursts of short high-frequency bipolar pulses could alleviate these problems. Therefore, we designed our study to verify if it is possible to use high-frequency bipolar pulses (HF-EP pulses) in electrochemotherapy. Materials and methods We performed in vitro experiments on mouse skin melanoma (B16-F1) cells by adding 1-330 μM cisplatin and delivering either (a) eight 100 μs long monopolar pulses, 0.4-1.2 kV/cm, 1 Hz (ECT pulses) or (b) eight bursts at 1 Hz, consisting of 50 bipolar pulses. One bipolar pulse consisted of a series of 1 μs long positive and 1 μs long negative pulse (0.5-5 kV/cm) with a 1 μs delay in-between. Results With both types of pulses, the combination of electric pulses and cisplatin was more efficient in killing cells than cisplatin or electric pulses only. However, we needed to apply a higher electric field in HF-EP (3 kV/cm) than in ECT (1.2 kV/cm) to obtain comparable cytotoxicity. Conclusions It is possible to use HF-EP in electrochemotherapy; however, at the expense of applying higher electric fields than in classical ECT. The results obtained, nevertheless, offer an evidence that HF-EP could be used in electrochemotherapy with potentially alleviated muscle contractions and pain.
Keywords: cell survival; cisplatin; drug uptake; electrochemotherapy; electroporation; high-frequency bipolar pulses.
Figures

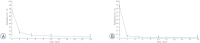
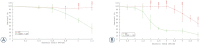

Similar articles
-
The equivalence of different types of electric pulses for electrochemotherapy with cisplatin - an in vitro study.Radiol Oncol. 2024 Feb 21;58(1):51-66. doi: 10.2478/raon-2024-0005. eCollection 2024 Mar 1. Radiol Oncol. 2024. PMID: 38378034 Free PMC article.
-
The Effects of Bipolar Cancellation Phenomenon on Nano-Electrochemotherapy of Melanoma Tumors: In Vitro and In Vivo Pilot.Int J Mol Sci. 2024 Aug 28;25(17):9338. doi: 10.3390/ijms25179338. Int J Mol Sci. 2024. PMID: 39273287 Free PMC article.
-
Connecting the in vitro and in vivo experiments in electrochemotherapy - a feasibility study modeling cisplatin transport in mouse melanoma using the dual-porosity model.J Control Release. 2018 Sep 28;286:33-45. doi: 10.1016/j.jconrel.2018.07.021. Epub 2018 Jul 20. J Control Release. 2018. PMID: 30016733
-
Microsecond and nanosecond electric pulses in cancer treatments.Bioelectromagnetics. 2012 Feb;33(2):106-23. doi: 10.1002/bem.20692. Epub 2011 Aug 3. Bioelectromagnetics. 2012. PMID: 21812011 Review.
-
[Progress in electrochemotherapy].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2004 Dec;21(6):1043-6. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2004. PMID: 15646362 Review. Chinese.
Cited by
-
The equivalence of different types of electric pulses for electrochemotherapy with cisplatin - an in vitro study.Radiol Oncol. 2024 Feb 21;58(1):51-66. doi: 10.2478/raon-2024-0005. eCollection 2024 Mar 1. Radiol Oncol. 2024. PMID: 38378034 Free PMC article.
-
Image Analysis of 3D Conjunctival Melanoma Cell Cultures Following Electrochemotherapy.Biomedicines. 2020 Jun 13;8(6):158. doi: 10.3390/biomedicines8060158. Biomedicines. 2020. PMID: 32545782 Free PMC article.
-
Threshold Interphase Delay for Bipolar Pulses to Prevent Cancellation Phenomenon during Electrochemotherapy.Int J Mol Sci. 2024 Aug 12;25(16):8774. doi: 10.3390/ijms25168774. Int J Mol Sci. 2024. PMID: 39201461 Free PMC article.
-
Short microsecond pulses achieve homogeneous electroporation of elongated biological cells irrespective of their orientation in electric field.Sci Rep. 2020 Jun 4;10(1):9149. doi: 10.1038/s41598-020-65830-3. Sci Rep. 2020. PMID: 32499601 Free PMC article.
-
Muscle contractions and pain sensation accompanying high-frequency electroporation pulses.Sci Rep. 2022 May 16;12(1):8019. doi: 10.1038/s41598-022-12112-9. Sci Rep. 2022. PMID: 35577873 Free PMC article.
References
-
- Kotnik T, Kramar P, Pucihar G, Miklavcic D, Tarek M. Cell membrane electroporation-part 1: the phenomenon. IEEE Electr Insul Mag. 2012;28:14–23. 10.1109/MEI.2012.6268438 - DOI
-
- Weaver JC. Electroporation: a general phenomenon for manipulating cells and tissues. J Cell Biochem. 1993;51:426–35. 10.1002/jcb.2400510407 - DOI - PubMed
-
- Tsong TY. Electroporation of cell membranes. Biophys J. 1991;60:297–306. 10.1016/S0006-3495(91)82054-9 - DOI - PMC - PubMed
-
- Kotnik T, Rems L, Tarek M, Miklavčič D. Membrane electroporation and electropermeabilization: mechanisms and models. Annu Rev Biophys. 2019;48 10.1146/annurev-biophys-052118-115451 - DOI - PubMed
-
- Yarmush ML, Golberg A, Serša G, Kotnik T, Miklavčič D. Electroporation-based technologies for medicine: principles, applications, and challenges. Annu Rev Biomed Eng. 2014;16:295–320. 10.1146/annurev-bio-eng-071813-104622 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
