Dengue and Zika Virus Diagnostic Testing for Patients with a Clinically Compatible Illness and Risk for Infection with Both Viruses
- PMID: 31194720
- PMCID: PMC6581290
- DOI: 10.15585/mmwr.rr6801a1
Dengue and Zika Virus Diagnostic Testing for Patients with a Clinically Compatible Illness and Risk for Infection with Both Viruses
Abstract
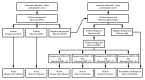
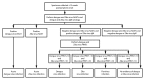
Dengue and Zika viruses are closely related mosquitoborne flaviviruses with similar transmission cycles, distribution throughout the tropics and subtropics, and disease manifestations including fever, rash, myalgia, and arthralgia. For patients with suspected dengue or Zika virus disease, nucleic acid amplification tests (NAATs) are the preferred method of diagnosis. Immunoglobulin M (IgM) antibody testing can identify additional infections and remains an important tool for the diagnosis of these diseases, but interpreting the results is complicated by cross-reactivity, and determining the specific timing of infection can be difficult. These limitations are a particular challenge for pregnant women in determining whether Zika virus infection occurred during or before the pregnancy.This report summarizes existing and new guidance on dengue and Zika virus diagnostic testing for patients with a clinically compatible illness who live in or recently traveled to an area where there is risk for infection with both viruses. CDC recommendations for screening of asymptomatic pregnant women with possible Zika virus exposure are unchanged. For symptomatic nonpregnant persons, dengue and Zika virus NAATs should be performed on serum collected ≤7 days after symptom onset. Dengue and Zika virus IgM antibody testing should be performed on NAAT-negative serum specimens or serum collected >7 days after onset of symptoms. For symptomatic pregnant women, serum and urine specimens should be collected as soon as possible within 12 weeks of symptom onset for concurrent dengue and Zika virus NAATs and IgM antibody testing. Positive IgM antibody test results with negative NAAT results should be confirmed by neutralizing antibody tests when clinically or epidemiologically indicated, including for all pregnant women. Data on the epidemiology of viruses known to be circulating at the location of exposure and clinical findings should be considered when deciding which tests to perform and for interpreting results.Patients with clinically suspected dengue should receive appropriate management to monitor and treat shock and hemorrhage. Women with laboratory evidence of possible Zika virus infection during pregnancy and their infants should be evaluated and managed for possible adverse outcomes. Dengue and Zika virus disease are nationally notifiable conditions, and cases should be reported to public health authorities.
Conflict of interest statement
No conflicts of interest were reported.
Figures
Similar articles
-
Update: Interim Guidance for Health Care Providers Caring for Pregnant Women with Possible Zika Virus Exposure - United States, July 2016.MMWR Morb Mortal Wkly Rep. 2016 Jul 25;65(29):739-44. doi: 10.15585/mmwr.mm6529e1. MMWR Morb Mortal Wkly Rep. 2016. PMID: 27467820
-
Interim Guidance for Interpretation of Zika Virus Antibody Test Results.MMWR Morb Mortal Wkly Rep. 2016 Jun 3;65(21):543-6. doi: 10.15585/mmwr.mm6521e1. MMWR Morb Mortal Wkly Rep. 2016. PMID: 27254248
-
Patterns in Zika Virus Testing and Infection, by Report of Symptoms and Pregnancy Status - United States, January 3-March 5, 2016.MMWR Morb Mortal Wkly Rep. 2016 Apr 22;65(15):395-9. doi: 10.15585/mmwr.mm6515e1. MMWR Morb Mortal Wkly Rep. 2016. PMID: 27101541
-
Testing for Zika virus infection in pregnancy: key concepts to deal with an emerging epidemic.Am J Obstet Gynecol. 2017 Mar;216(3):209-225. doi: 10.1016/j.ajog.2017.01.020. Epub 2017 Jan 23. Am J Obstet Gynecol. 2017. PMID: 28126366 Review.
-
Epidemiology and neurological complications of infection by the Zika virus: a new emerging neurotropic virus.Rev Neurol. 2016 Apr 1;62(7):317-28. Rev Neurol. 2016. PMID: 26988170 Review. English, Spanish.
Cited by
-
Zika Virus Infection Knowledge and Communication Preferences Among Women of Reproductive Age in Central Brooklyn, New York: A Thematic Analysis.J Community Health. 2024 Dec;49(6):1044-1053. doi: 10.1007/s10900-024-01365-2. Epub 2024 Jun 2. J Community Health. 2024. PMID: 38824473
-
Neuroinvasion of emerging and re-emerging arboviruses: A scoping review.SAGE Open Med. 2024 May 6;12:20503121241229847. doi: 10.1177/20503121241229847. eCollection 2024. SAGE Open Med. 2024. PMID: 38711470 Free PMC article.
-
Nervous System Manifestations of Arboviral Infections.Curr Trop Med Rep. 2022;9(4):107-118. doi: 10.1007/s40475-022-00262-9. Epub 2022 Sep 15. Curr Trop Med Rep. 2022. PMID: 36124288 Free PMC article. Review.
-
Brazilian Protocol for Sexually Transmitted Infections 2020: Zika virus infection.Rev Soc Bras Med Trop. 2021 May 17;54(suppl 1):e2020609. doi: 10.1590/0037-8682-609-2020. eCollection 2021. Rev Soc Bras Med Trop. 2021. PMID: 34008724 Free PMC article.
-
Risk factors for infection with chikungunya and Zika viruses in southern Puerto Rico: A community-based cross-sectional seroprevalence survey.PLoS Negl Trop Dis. 2022 Jun 13;16(6):e0010416. doi: 10.1371/journal.pntd.0010416. eCollection 2022 Jun. PLoS Negl Trop Dis. 2022. PMID: 35696355 Free PMC article.
References
-
- Simmons CP, Farrar JJ, Nguyen V, Wills B. Dengue. N Engl J Med 2012;366:1423–32. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
