Development of a physiologically-based pharmacokinetic pediatric brain model for prediction of cerebrospinal fluid drug concentrations and the influence of meningitis
- PMID: 31194730
- PMCID: PMC6592555
- DOI: 10.1371/journal.pcbi.1007117
Development of a physiologically-based pharmacokinetic pediatric brain model for prediction of cerebrospinal fluid drug concentrations and the influence of meningitis
Abstract
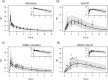
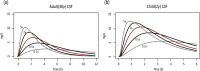
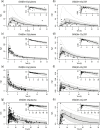
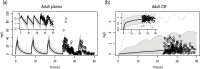
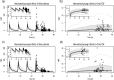
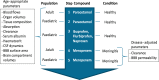
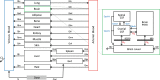
Different pediatric physiologically-based pharmacokinetic (PBPK) models have been described incorporating developmental changes that influence plasma drug concentrations. Drug disposition into cerebrospinal fluid (CSF) is also subject to age-related variation and can be further influenced by brain diseases affecting blood-brain barrier integrity, like meningitis. Here, we developed a generic pediatric brain PBPK model to predict CSF concentrations of drugs that undergo passive transfer, including age-appropriate parameters. The model was validated for the analgesics paracetamol, ibuprofen, flurbiprofen and naproxen, and for a pediatric meningitis population by empirical optimization of the blood-brain barrier penetration of the antibiotic meropenem. Plasma and CSF drug concentrations derived from the literature were used to perform visual predictive checks and to calculate ratios between simulated and observed area under the concentration curves (AUCs) in order to evaluate model performance. Model-simulated concentrations were comparable to observed data over a broad age range (3 months-15 years postnatal age) for all drugs investigated. The ratios between observed and simulated AUCs (AUCo/AUCp) were within 2-fold difference both in plasma (range 0.92-1.09) and in CSF (range 0.64-1.23) indicating acceptable model performance. The model was also able to describe disease-mediated changes in neonates and young children (<3m postnatal age) related to meningitis and sepsis (range AUCo/AUCp plasma: 1.64-1.66, range AUCo/AUCp CSF: 1.43-1.73). Our model provides a new computational tool to predict CSF drug concentrations in children with and without meningitis and can be used as a template model for other compounds that passively enter the CNS.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

