Comparative effectiveness of second generation long-acting injectable antipsychotics based on nationwide database research in Hungary
- PMID: 31194778
- PMCID: PMC6563992
- DOI: 10.1371/journal.pone.0218071
Comparative effectiveness of second generation long-acting injectable antipsychotics based on nationwide database research in Hungary
Abstract
Background: Schizophrenia is a severe condition that affects approximately 1% of the population. Certain elements of antipsychotic treatment can only be examined in large population, thus the need for population-based real-world analyses has been increasing.
Patients and methods: Hungarian National Health Fund database includes all healthcare data of the population of Hungary. All patients diagnosed with schizophrenia between 01.01.2006 and 31.12.2015 were included in the study. We analyzed all patients with newly initiated second-generation antipsychotic during the inclusion period (01.01.2012-31.12.2013). Patients were followed for 2 years. All-cause treatment discontinuation served as the primary outcome of the study. Patients with newly initiated long-acting injectable treatments were further investigated in stratified analyses based on their previous treatment.
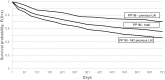
Results: 106,624 patients had schizophrenia diagnosis during the study period. 12,232 patients met the inclusion criteria for newly initiating second-generation antipsychotic during the inclusion period. The proportion of patients still on treatment after 1 year for oral treatments varied between 17% (oral risperidone) and 31% (oral olanzapine) while the analogous data for long acting injectables were between 32% (risperidone long acting) and 64% (paliperidone long acting one monthly). The 2-year data were similarly in favor of long-actings. Median time to discontinuation in the oral group varied between 57 days (clozapine) and 121 days (olanzapine). The median time to discontinuation for long-actings was significantly longer: between 176 and 287 days; in case of paliperidone long acting, median was not reached during the observation period. Patients receiving long-acting treatment switched from another long-acting remained on the newly initiated treatment significantly longer than those switched from orals.
Conclusion: Our results indicate the superiority of second generation long-acting antipsychotics with regard to rates of treatment discontinuation and periods of persistence to the assigned medication.
Conflict of interest statement
Four authors (P Takács, A Borsi, L Fehér and J Sermon) are full time employees of different affiliates of Janssen, a Johnson and Johnson Owned company. P. Takács and L. Feher are employees of Janssen-Cilag Kft. J Sermon is an employee of Janssen Pharmaceutica NV. A. Borsi is an employee of Janssen-Cilag Limited. All of the four Janssen employees hold common shares and Johnson and Johnson, the mother company of Janssen. Four authors (M Bacskai, P Rakonczai, R Hegyi and T Németh) were employees of a commercial organization at the time of the research- an independent data research company, Healthware Consulting Kft, which received funding for contribution to the study design and data analyses from Janssen. P Rakonczai is currently employed by Evidera Kft. P Fadgyas-Freyler and J Gimesi-Országh. were employees of the Hungarian National Health Insurance Fund (NHIF) at the time of the study. J Gimesi-Országh currently works as an independent consultant while P Fadgyas-Freyler is still an employee of NHIF. I Bitter has been an advisory board member/consultant/lecturer in the last 5 years for Angelini, EGIS, Eli Lilly, Janssen, Lundbeck, Pierre Fabré, Gedeon Richter and Servier. These competing interests do not alter our adherence to PLOS ONE policies on sharing data and materials. There are no patents, products in development or marketed products to declare. All other authors declare no competing interests.
Figures
Similar articles
-
Comparative Effectiveness of Second Generation Long-acting Injectable Antipsychotics Based on Nationwide Database Research in Hungary: An Update.Schizophr Bull Open. 2022 Jan 31;3(1):sgac013. doi: 10.1093/schizbullopen/sgac013. eCollection 2022 Jan. Schizophr Bull Open. 2022. PMID: 39144770 Free PMC article.
-
Real-World Effectiveness of Antipsychotic Treatments in a Nationwide Cohort of 29 823 Patients With Schizophrenia.JAMA Psychiatry. 2017 Jul 1;74(7):686-693. doi: 10.1001/jamapsychiatry.2017.1322. JAMA Psychiatry. 2017. PMID: 28593216 Free PMC article.
-
Healthcare resource utilization of second-generation long-acting injectable antipsychotics in schizophrenia: risperidone versus paliperidone palmitate.Curr Med Res Opin. 2016 Nov;32(11):1873-1881. doi: 10.1080/03007995.2016.1219706. Epub 2016 Aug 22. Curr Med Res Opin. 2016. PMID: 27479694
-
New second-generation long-acting injectable antipsychotics for the treatment of schizophrenia.Expert Rev Neurother. 2013 Jul;13(7):767-83. doi: 10.1586/14737175.2013.811984. Expert Rev Neurother. 2013. PMID: 23898849 Review.
-
Long-acting injectable formulations of antipsychotic drugs for the treatment of schizophrenia.Arch Pharm Res. 2013 Jun;36(6):651-9. doi: 10.1007/s12272-013-0105-7. Epub 2013 Apr 1. Arch Pharm Res. 2013. PMID: 23543652 Review.
Cited by
-
The Effect of Longer Dosing Intervals for Long-Acting Injectable Antipsychotics on Outcomes in Schizophrenia.Neuropsychiatr Dis Treat. 2023 Mar 7;19:531-545. doi: 10.2147/NDT.S395383. eCollection 2023. Neuropsychiatr Dis Treat. 2023. PMID: 36915909 Free PMC article. Review.
-
A meta-analysis of effectiveness of real-world studies of antipsychotics in schizophrenia: Are the results consistent with the findings of randomized controlled trials?Transl Psychiatry. 2021 Oct 6;11(1):510. doi: 10.1038/s41398-021-01636-9. Transl Psychiatry. 2021. PMID: 34615850 Free PMC article. Review.
-
Comparative Effectiveness of Second Generation Long-acting Injectable Antipsychotics Based on Nationwide Database Research in Hungary: An Update.Schizophr Bull Open. 2022 Jan 31;3(1):sgac013. doi: 10.1093/schizbullopen/sgac013. eCollection 2022 Jan. Schizophr Bull Open. 2022. PMID: 39144770 Free PMC article.
-
Three-Year Outcomes of 6-Month Paliperidone Palmitate in Adults With Schizophrenia: An Open-Label Extension Study of a Randomized Clinical Trial.JAMA Netw Open. 2024 Jul 1;7(7):e2421495. doi: 10.1001/jamanetworkopen.2024.21495. JAMA Netw Open. 2024. PMID: 39018073 Free PMC article. Clinical Trial.
-
Comparative effectiveness study of paliperidone palmitate 6-month with a real-world external comparator arm of paliperidone palmitate 1-month or 3-month in patients with schizophrenia.Ther Adv Psychopharmacol. 2023 Sep 29;13:20451253231200258. doi: 10.1177/20451253231200258. eCollection 2023. Ther Adv Psychopharmacol. 2023. PMID: 37786804 Free PMC article.
References
-
- Park SC, Choi MY, Choi J, Park E, Tchoe HJ, Suh JK, et al. Comparative Efficacy and Safety of Long-acting Injectable and Oral Second-generation Antipsychotics for the Treatment of Schizophrenia: A Systematic Review and Meta-analysis. Clin Psychopharmacol Neurosci. 2018. November 30;16(4):361–375. 10.9758/cpn.2018.16.4.361 - DOI - PMC - PubMed
-
- Stroup T, McEvoy J, Swartz M, Byerly M, Glick I, Canive J et al. The National Institute of Mental Health Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) project: schizophrenia trial design and protocol development. Schizophr Bull. 2003;29(1):15–31. 10.1093/oxfordjournals.schbul.a006986 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous