Systemically transplanted mesenchymal stem cells induce vascular-like structure formation in a rat model of vaginal injury
- PMID: 31194823
- PMCID: PMC6563972
- DOI: 10.1371/journal.pone.0218081
Systemically transplanted mesenchymal stem cells induce vascular-like structure formation in a rat model of vaginal injury
Abstract
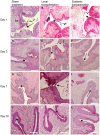
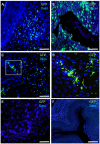
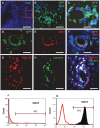
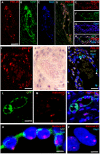
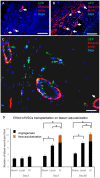
The beneficial effect of mesenchymal stem cells (MSCs) on wound healing is mostly attributed to a trophic effect that promotes angiogenesis. Whether MSCs can contribute to the formation of new blood vessels by direct differentiation is still controversial. Pelvic floor dysfunction (PFD) is a group of disorders that negatively affect the quality of women's lives. Traditional vaginal surgical repair provides disappointing anatomical outcome. Stem cell transplantation may be used to supplement surgery and improve its outcome. Here we aimed to examine the engraftment, survival, differentiation and angiogenic effect of transplanted MSCs in a vaginal injury rat model. MSCs were obtained from the bone marrow of Sprague Drawley (SD) rats, expanded and characterized in vitro. The MSCs expressed CD90 and CD29, did not express CD45, CD34, CD11b and CD31 and could differentiate into osteogenic, chondrogenic and adipogenic lineages. Cells were labeled with either PKH-26 or GFP and transplanted systemically or locally to female SD rats, just after a standardized vaginal incision was made. Engraftment after local transplantation was less efficient at all-time points compared to systemic administration. In the systemically transplanted animal group, MSCs migrated to the injury site and were present in the healed vagina for at least 30 days. Both systemic and local MSCs transplantation promoted host angiogenesis. Systemically transplanted MSCs created new vascular-like structures by direct differentiation into endothelium. These findings pave the way to further studies of the potential role of MSCs transplantation in improving surgical outcome in women with PFD.
Conflict of interest statement
We declare that no competing interests exist. Benjamin Reubinoff is a founder, holds shares and is the Chief Scientific Officer of CellCure Neuroscience Ltd. The focus of the company is the development of human embryonic stem cells for transplantation therapy in neurological and retinal degeneration disorders. The company did not fund the study presented in this manuscript and has no interest in its results. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
Similar articles
-
Transplantation of Mesenchymal Stem Cells Derived from Old Rats Improves Healing and Biomechanical Properties of Vaginal Tissue Following Surgical Incision in Aged Rats.Int J Mol Sci. 2024 May 24;25(11):5714. doi: 10.3390/ijms25115714. Int J Mol Sci. 2024. PMID: 38891914 Free PMC article.
-
Mesenchymal stem cell transplantation improves biomechanical properties of vaginal tissue following full-thickness incision in aged rats.Stem Cell Reports. 2022 Nov 8;17(11):2565-2578. doi: 10.1016/j.stemcr.2022.09.005. Epub 2022 Oct 13. Stem Cell Reports. 2022. PMID: 36240774 Free PMC article.
-
Mesenchymal stem cell transplantation in a rabbit corneal alkali burn model: engraftment and involvement in wound healing.Eye (Lond). 2006 Apr;20(4):482-90. doi: 10.1038/sj.eye.6701913. Eye (Lond). 2006. PMID: 15895027
-
Rat mesenchymal stem cell secretome promotes elastogenesis and facilitates recovery from simulated childbirth injury.Cell Transplant. 2014;23(11):1395-406. doi: 10.3727/096368913X670921. Epub 2013 Jul 17. Cell Transplant. 2014. PMID: 23866688 Free PMC article.
-
Important role of the SDF-1/CXCR4 axis in the homing of systemically transplanted human amnion-derived mesenchymal stem cells (hAD-MSCs) to ovaries in rats with chemotherapy-induced premature ovarian insufficiency (POI).Stem Cell Res Ther. 2022 Feb 23;13(1):79. doi: 10.1186/s13287-022-02759-6. Stem Cell Res Ther. 2022. PMID: 35197118 Free PMC article.
Cited by
-
Ex vivo-expanded highly pure ABCB5+ mesenchymal stromal cells as Good Manufacturing Practice-compliant autologous advanced therapy medicinal product for clinical use: process validation and first in-human data.Cytotherapy. 2021 Feb;23(2):165-175. doi: 10.1016/j.jcyt.2020.08.012. Epub 2020 Oct 1. Cytotherapy. 2021. PMID: 33011075 Free PMC article.
-
CD146+Mesenchymal stem cells treatment improves vascularization, muscle contraction and VEGF expression, and reduces apoptosis in rat ischemic hind limb.Biochem Pharmacol. 2021 Aug;190:114530. doi: 10.1016/j.bcp.2021.114530. Epub 2021 Apr 21. Biochem Pharmacol. 2021. PMID: 33891966 Free PMC article.
-
Transplantation of Mesenchymal Stem Cells Derived from Old Rats Improves Healing and Biomechanical Properties of Vaginal Tissue Following Surgical Incision in Aged Rats.Int J Mol Sci. 2024 May 24;25(11):5714. doi: 10.3390/ijms25115714. Int J Mol Sci. 2024. PMID: 38891914 Free PMC article.
-
Materials Selection for the Injection into Vaginal Wall for Treatment of Vaginal Atrophy.Aesthetic Plast Surg. 2021 Jun;45(3):1231-1241. doi: 10.1007/s00266-020-02054-w. Epub 2021 Mar 1. Aesthetic Plast Surg. 2021. PMID: 33649927 Review.
-
MSC-based therapy in female pelvic floor disorders.Cell Biosci. 2020 Sep 10;10:104. doi: 10.1186/s13578-020-00466-4. eCollection 2020. Cell Biosci. 2020. PMID: 32944218 Free PMC article. Review.
References
-
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999. April 2;284(5411):143–7. - PubMed
-
- Araña M, Mazo M, Aranda P, Pelacho B, Prosper F. Adipose tissue-derived mesenchymal stem cells: isolation, expansion, and characterization. Methods Mol Biol Clifton NJ. 2013;1036:47–61. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

