Effects of nicotine exposure on murine mandibular development
- PMID: 31194840
- PMCID: PMC6564027
- DOI: 10.1371/journal.pone.0218376
Effects of nicotine exposure on murine mandibular development
Abstract
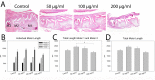
Nicotine is known to affect cell proliferation and differentiation, two processes vital to proper development of the mandible. The mandible, the lower jaw in mammals and fish, plays a crucial role in craniofacial development. Malformation of the jaw can precipitate a plethora of complications including disrupting development of the upper jaw, the palate, and or impeding airway function. The purpose of this study was to test the hypothesis that in utero nicotine exposure alters the development of the murine mandible in a dose dependent manner. To test this hypothesis, wild type C57BL6 mice were used to produce in utero nicotine exposed litters by adding nicotine to the drinking water of pregnant dams at concentrations of 0 μg/ml (control), 50 μg/ml (low), 100 μg/ml (medium), 200 μg/ml (high) throughout pregnancy to birth of litters mimicking clinically relevant nicotine exposures. Resultant pups revealed no significant differences in body weight however, cephalometric investigation revealed several dimensions affected by nicotine exposure including mandibular ramus height, mandibular body height, and molar length. Histological investigation of molars revealed an increase in proliferation and a decrease in apoptosis with nicotine exposure. These results demonstrate the direct effects of nicotine on the developing mandible outside the context of tobacco use, indicating that nicotine use including tobacco alternatives, cessation methods, and electronic nicotine delivering products may disrupt normal growth and development of the craniofacial complex.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
In utero nicotine exposure affects murine palate development.Orthod Craniofac Res. 2024 Dec;27(6):967-973. doi: 10.1111/ocr.12844. Epub 2024 Aug 2. Orthod Craniofac Res. 2024. PMID: 39092604
-
In utero exposure to selective serotonin re-uptake inhibitor affects murine mandibular development.Orthod Craniofac Res. 2023 Aug;26(3):415-424. doi: 10.1111/ocr.12624. Epub 2022 Dec 19. Orthod Craniofac Res. 2023. PMID: 36458927
-
Postnatal effects of nicotine on first molar development in the CD-1 mouse.Acta Anat (Basel). 1991;140(3):269-72. doi: 10.1159/000147067. Acta Anat (Basel). 1991. PMID: 1867069
-
[Cigarette smoke and nicotine during pregnancy : where are we today?].Rev Med Suisse. 2020 Feb 19;16(682):357-360. Rev Med Suisse. 2020. PMID: 32073770 Review. French.
-
Behavioral and neural consequences of prenatal exposure to nicotine.J Am Acad Child Adolesc Psychiatry. 2001 Jun;40(6):630-41. doi: 10.1097/00004583-200106000-00007. J Am Acad Child Adolesc Psychiatry. 2001. PMID: 11392340 Review.
Cited by
-
Exposure to maternal nicotine in utero and/or via lactation alters craniofacial development in mice.PLoS One. 2025 Aug 1;20(8):e0329403. doi: 10.1371/journal.pone.0329403. eCollection 2025. PLoS One. 2025. PMID: 40748875 Free PMC article.
-
Embryonic Nicotine Exposure Disrupts Adult Social Behavior and Craniofacial Development in Zebrafish.Toxics. 2022 Oct 15;10(10):612. doi: 10.3390/toxics10100612. Toxics. 2022. PMID: 36287892 Free PMC article.
-
Intrauterine exposure to nicotine through maternal vaping disrupts embryonic lung and skeletal development via the Kcnj2 potassium channel.Dev Biol. 2023 Sep;501:111-123. doi: 10.1016/j.ydbio.2023.06.002. Epub 2023 Jun 22. Dev Biol. 2023. PMID: 37353105 Free PMC article.
-
Manzamine-A Alters In Vitro Calvarial Osteoclast Function.J Nat Prod. 2024 Mar 22;87(3):560-566. doi: 10.1021/acs.jnatprod.3c01097. Epub 2024 Feb 21. J Nat Prod. 2024. PMID: 38383319 Free PMC article.
-
In utero exposure to electronic cigarette carriers alters craniofacial morphology.PLoS One. 2025 Jun 30;20(6):e0327190. doi: 10.1371/journal.pone.0327190. eCollection 2025. PLoS One. 2025. PMID: 40587499 Free PMC article.
References
-
- Schoenborn CA, Gindi RM. Electronic Cigarette Use Among Adults: United States, 2014. National Center for Health Statistics Data Brief. 2015;10(217):1–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

