Therapeutic efficacy of favipiravir against Bourbon virus in mice
- PMID: 31194854
- PMCID: PMC6564012
- DOI: 10.1371/journal.ppat.1007790
Therapeutic efficacy of favipiravir against Bourbon virus in mice
Abstract
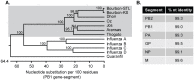
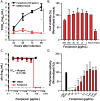
Bourbon virus (BRBV) is an emerging tick-borne RNA virus in the orthomyxoviridae family that was discovered in 2014. Although fatal human cases of BRBV have been described, little is known about its pathogenesis, and no antiviral therapies or vaccines exist. We obtained serum from a fatal case in 2017 and successfully recovered the second human infectious isolate of BRBV. Next-generation sequencing of the St. Louis isolate of BRBV (BRBV-STL) showed >99% nucleotide identity to the original reference isolate. Using BRBV-STL, we developed a small animal model to study BRBV-STL tropism in vivo and evaluated the prophylactic and therapeutic efficacy of the experimental antiviral drug favipiravir against BRBV-induced disease. Infection of Ifnar1-/- mice lacking the type I interferon receptor, but not congenic wild-type animals, resulted in uniformly fatal disease 6 to 10 days after infection. RNA in situ hybridization and viral yield assays demonstrated a broad tropism of BRBV-STL with highest levels detected in liver and spleen. In vitro replication and polymerase activity of BRBV-STL were inhibited by favipiravir. Moreover, administration of favipiravir as a prophylaxis or as post-exposure therapy three days after infection prevented BRBV-STL-induced mortality in immunocompromised Ifnar1-/- mice. These results suggest that favipiravir may be a candidate treatment for humans who become infected with BRBV.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Essential Role of Interferon Response in Containing Human Pathogenic Bourbon Virus.Emerg Infect Dis. 2019 Jul;25(7):1304-1313. doi: 10.3201/eid2507.181062. Emerg Infect Dis. 2019. PMID: 31211667 Free PMC article.
-
Eight years' advances on Bourbon virus, a tick-born Thogotovirus of the Orthomyxovirus family.Zoonoses. 2022 Jan 6;2(1):18. doi: 10.15212/zoonoses-2022-0012. Epub 2022 Jun 14. Zoonoses. 2022. PMID: 35727718 Free PMC article.
-
Favipiravir Inhibits Mayaro Virus Infection in Mice.Viruses. 2021 Nov 3;13(11):2213. doi: 10.3390/v13112213. Viruses. 2021. PMID: 34835018 Free PMC article.
-
Favipiravir as a potential countermeasure against neglected and emerging RNA viruses.Antiviral Res. 2018 May;153:85-94. doi: 10.1016/j.antiviral.2018.03.003. Epub 2018 Mar 7. Antiviral Res. 2018. PMID: 29524445 Review.
-
[Favipiravir, a new concept of antiviral drug against influenza viruses].Rev Esp Quimioter. 2017 Apr;30(2):79-83. Epub 2017 Feb 8. Rev Esp Quimioter. 2017. PMID: 28176519 Review. Spanish.
Cited by
-
Evidence of Human Bourbon Virus Infections, North Carolina, USA.Emerg Infect Dis. 2024 Nov;30(11):2396-2399. doi: 10.3201/eid3011.240499. Epub 2024 Oct 9. Emerg Infect Dis. 2024. PMID: 39387510 Free PMC article.
-
Prevalence of Bourbon and Heartland viruses in field collected ticks at an environmental field station in St. Louis County, Missouri, USA.Ticks Tick Borne Dis. 2023 Jan;14(1):102080. doi: 10.1016/j.ttbdis.2022.102080. Epub 2022 Nov 9. Ticks Tick Borne Dis. 2023. PMID: 36375268 Free PMC article.
-
Comparative Study of Ten Thogotovirus Isolates and Their Distinct In Vivo Characteristics.J Virol. 2022 Mar 9;96(5):e0155621. doi: 10.1128/JVI.01556-21. Epub 2022 Jan 12. J Virol. 2022. PMID: 35019718 Free PMC article.
-
Diversification of Bourbon Virus in New York State.Microorganisms. 2023 Jun 15;11(6):1590. doi: 10.3390/microorganisms11061590. Microorganisms. 2023. PMID: 37375092 Free PMC article.
-
Human Tick-Borne Diseases and Advances in Anti-Tick Vaccine Approaches: A Comprehensive Review.Vaccines (Basel). 2024 Jan 29;12(2):141. doi: 10.3390/vaccines12020141. Vaccines (Basel). 2024. PMID: 38400125 Free PMC article. Review.
References
-
- Lambert AJ, Velez JO, Brault AC, Calvert AE, Bell-Sakyi L, Bosco-Lauth AM, et al. Molecular, serological and in vitro culture-based characterization of Bourbon virus, a newly described human pathogen of the genus Thogotovirus. J Clin Virol. 2015;73:127–32. 10.1016/j.jcv.2015.10.021 Epub Oct 27. - DOI - PMC - PubMed
-
- Savage HM, Godsey MS Jr., Panella NA, Burkhalter KL, Manford J, Trevino-Garrison IC, et al. Surveillance for Tick-Borne Viruses Near the Location of a Fatal Human Case of Bourbon Virus (Family Orthomyxoviridae: Genus Thogotovirus) in Eastern Kansas, 2015. Journal of medical entomology. 2018;55(3):701–5. Epub 2018/01/25. 10.1093/jme/tjx251 . - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

