Regulation of arginine transport by GCN2 eIF2 kinase is important for replication of the intracellular parasite Toxoplasma gondii
- PMID: 31194856
- PMCID: PMC6564765
- DOI: 10.1371/journal.ppat.1007746
Regulation of arginine transport by GCN2 eIF2 kinase is important for replication of the intracellular parasite Toxoplasma gondii
Abstract
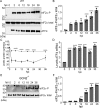
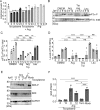
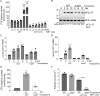
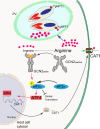
Toxoplasma gondii is a prevalent protozoan parasite that can infect any nucleated cell but cannot replicate outside of its host cell. Toxoplasma is auxotrophic for several nutrients including arginine, tryptophan, and purines, which it must acquire from its host cell. The demands of parasite replication rapidly deplete the host cell of these essential nutrients, yet Toxoplasma successfully manages to proliferate until it lyses the host cell. In eukaryotic cells, nutrient starvation can induce the integrated stress response (ISR) through phosphorylation of an essential translation factor eIF2. Phosphorylation of eIF2 lowers global protein synthesis coincident with preferential translation of gene transcripts involved in stress adaptation, such as that encoding the transcription factor ATF4 (CREB2), which activates genes that modulate amino acid metabolism and uptake. Here, we discovered that the ISR is induced in host cells infected with Toxoplasma. Our results show that as Toxoplasma depletes host cell arginine, the host cell phosphorylates eIF2 via protein kinase GCN2 (EIF2AK4), leading to induced ATF4. Increased ATF4 then enhances expression of the cationic amino acid transporter CAT1 (SLC7A1), resulting in increased uptake of arginine in Toxoplasma-infected cells. Deletion of host GCN2, or its downstream effectors ATF4 and CAT1, lowers arginine levels in the host, impairing proliferation of the parasite. Our findings establish that Toxoplasma usurps the host cell ISR to help secure nutrients that it needs for parasite replication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Saliba KJ, Kirk K (2001) Nutrient acquisition by intracellular apicomplexan parasites: staying in for dinner. Int J Parasitol 31: 1321–1330. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

