A mouse model of Proteus syndrome
- PMID: 31194862
- PMCID: PMC6736390
- DOI: 10.1093/hmg/ddz116
A mouse model of Proteus syndrome
Abstract
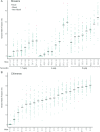
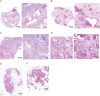
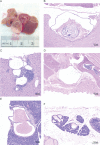
Proteus syndrome is a mosaic, progressive overgrowth disorder caused by a somatic activating variant c.49G > A p.(E17K) in AKT1. The presentation in affected individuals is variable, with a diversity of tissues demonstrating abnormalities. Common manifestations include skin and bony overgrowth, vascular malformations (VMs), cysts and benign tumors. We used two methods to create mouse models that had endogenously-regulated mosaic expression of the Proteus syndrome variant. Variant allele fractions (VAFs) ranged from 0% to 50% across numerous tissues in 44 Proteus syndrome mice. Mice were phenotypically heterogeneous with lesions rarely observed before 12 months of age. VMs were the most frequent finding with a total of 69 found in 29 of 44 Proteus syndrome mice. Twenty-eight cysts and ectasia, frequently biliary, were seen in 22 of 44 Proteus syndrome mice. Varying levels of mammary hyperplasia were seen in 10 of 16 female Proteus syndrome mice with other localized regions of hyperplasia and stromal expansion noted in several additional animals. Interestingly, 27 of 31 Proteus syndrome animals had non-zero blood VAF that is in contrast to the human disorder where it is rarely seen in peripheral blood. Identification of variant-positive cells by green fluorescent protein (GFP) staining in chimeric Proteus syndrome mice showed that in some lesions, hyperplastic cells were predominantly GFP/Akt1E17K-positive and showed increased pAKT signal compared to GFP-negative cells. However, hyperplastic mammary epithelium was a mixture of GFP/Akt1E17K-positive and negative cells with some GFP/Akt1E17K-negative cells also having increased pAKT signal suggesting that the variant-positive cells can induce lesion formation in a non-cell autonomous manner.
Published by Oxford University Press 2019.
Figures


References
-
- Biesecker L. (2006) The challenges of Proteus syndrome: diagnosis and management. Eur. J. Hum. Genet., 14, 1151–1157. - PubMed
-
- Biesecker L.G., Happle R., Mulliken J.B., Weksberg R., Graham J.M. Jr., Viljoen D.L. and Cohen M.M. Jr. (1999) Proteus syndrome: diagnostic criteria, differential diagnosis, and patient evaluation. Am. J. Med. Genet., 84, 389–395. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

