Inhaled corticosteroids in children with persistent asthma: effects of different drugs and delivery devices on growth
- PMID: 31194879
- PMCID: PMC6564081
- DOI: 10.1002/14651858.CD010126.pub2
Inhaled corticosteroids in children with persistent asthma: effects of different drugs and delivery devices on growth
Abstract
Background: Inhaled corticosteroids (ICS) are the most effective treatment for children with persistent asthma. Although treatment with ICS is generally considered to be safe in children, the potential adverse effects of these drugs on growth remains a matter of concern for parents and physicians.
Objectives: To assess the impact of different inhaled corticosteroid drugs and delivery devices on the linear growth of children with persistent asthma.
Search methods: We searched the Cochrane Airways Trials Register, which is derived from systematic searches of bibliographic databases including CENTRAL, MEDLINE, Embase, CINAHL, AMED and PsycINFO. We handsearched respiratory journals and meeting abstracts. We also conducted a search of ClinicalTrials.gov and manufacturers' clinical trial databases, or contacted the manufacturer, to search for potential relevant unpublished studies. The literature search was initially conducted in September 2014, and updated in November 2015, September 2018, and April 2019.
Selection criteria: We selected parallel-group randomized controlled trials of at least three months' duration. To be included, trials had to compare linear growth between different inhaled corticosteroid molecules at equivalent doses, delivered by the same type of device, or between different devices used to deliver the same inhaled corticosteroid molecule at the same dose, in children up to 18 years of age with persistent asthma.
Data collection and analysis: At least two review authors independently selected studies and assessed risk of bias in included studies. The data were extracted by one author and checked by another. The primary outcome was linear growth velocity. We conducted meta-analyses using Review Manager 5.3 software. We used mean differences (MDs) and 95% confidence intervals (CIs ) as the metrics for treatment effects, and the random-effects model for meta-analyses. We did not perform planned subgroup analyses due to there being too few included trials.
Main results: We included six randomized trials involving 1199 children aged from 4 to 12 years (per-protocol population: 1008), with mild-to-moderate persistent asthma. Two trials were from single hospitals, and the remaining four trials were multicentre studies. The duration of trials varied from six to 20 months.One trial with 23 participants compared fluticasone with beclomethasone, and showed that fluticasone given at an equivalent dose was associated with a significant greater linear growth velocity (MD 0.81 cm/year, 95% CI 0.46 to 1.16, low certainty evidence). Three trials compared fluticasone with budesonide. Fluticasone given at an equivalent dose had a less suppressive effect than budesonide on growth, as measured by change in height over a period from 20 weeks to 12 months (MD 0.97 cm, 95% CI 0.62 to 1.32; 2 trials, 359 participants; moderate certainty evidence). However, we observed no significant difference in linear growth velocity between fluticasone and budesonide at equivalent doses (MD 0.39 cm/year, 95% CI -0.94 to 1.73; 2 trials, 236 participants; very low certainty evidence).Two trials compared inhalation devices. One trial with 212 participants revealed a comparable linear growth velocity between beclomethasone administered via hydrofluoroalkane-metered dose inhaler (HFA-MDI) and beclomethasone administered via chlorofluorocarbon-metered dose inhaler (CFC-MDI) at an equivalent dose (MD -0.44 cm/year, 95% CI -1.00 to 0.12; low certainty evidence). Another trial with 229 participants showed a small but statistically significant greater increase in height over a period of six months in favour of budesonide via Easyhaler, compared to budesonide given at the same dose via Turbuhaler (MD 0.37 cm, 95% CI 0.12 to 0.62; low certainty evidence).
Authors' conclusions: This review suggests that the drug molecule and delivery device may impact the effect size of ICS on growth in children with persistent asthma. Fluticasone at an equivalent dose seems to inhibit growth less than beclomethasone and budesonide. Easyhaler is likely to have less adverse effect on growth than Turbuhaler when used for delivery of budesonide. However, the evidence from this systematic review of head-to-head trials is not certain enough to inform the selection of inhaled corticosteroid or inhalation device for the treatment of children with persistent asthma. Further studies are needed, and pragmatic trials and real-life observational studies seem more attractive and feasible.
Conflict of interest statement
IA: none known. EN: none known. SOP: none known. LZ: none known.
Figures



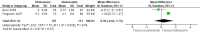
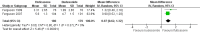



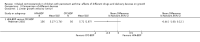

Update of
- doi: 10.1002/14651858.CD010126
Similar articles
-
Inhaled corticosteroids in children with persistent asthma: effects on growth.Cochrane Database Syst Rev. 2014 Jul 17;2014(7):CD009471. doi: 10.1002/14651858.CD009471.pub2. Cochrane Database Syst Rev. 2014. PMID: 25030198 Free PMC article.
-
Inhaled corticosteroids in children with persistent asthma: effects on growth.Evid Based Child Health. 2014 Dec;9(4):829-930. doi: 10.1002/ebch.1988. Evid Based Child Health. 2014. PMID: 25504972
-
Inhaled corticosteroids in children with persistent asthma: dose-response effects on growth.Evid Based Child Health. 2014 Dec;9(4):931-1046. doi: 10.1002/ebch.1989. Evid Based Child Health. 2014. PMID: 25504973
-
Inhaled corticosteroids in children with persistent asthma: dose-response effects on growth.Cochrane Database Syst Rev. 2014 Jul 17;2014(7):CD009878. doi: 10.1002/14651858.CD009878.pub2. Cochrane Database Syst Rev. 2014. PMID: 25030199 Free PMC article.
-
Regular treatment with formoterol and an inhaled corticosteroid versus regular treatment with salmeterol and an inhaled corticosteroid for chronic asthma: serious adverse events.Cochrane Database Syst Rev. 2021 Apr 14;4(4):CD007694. doi: 10.1002/14651858.CD007694.pub3. Cochrane Database Syst Rev. 2021. PMID: 33852162 Free PMC article.
Cited by
-
Long non-coding RNA (LncRNA) non-coding RNA activated by DNA damage (NORAD) knockdown alleviates airway remodeling in asthma via regulating miR-410-3p/RCC2 and inhibiting Wnt/β-catenin pathway.Heliyon. 2023 Dec 15;10(1):e23860. doi: 10.1016/j.heliyon.2023.e23860. eCollection 2024 Jan 15. Heliyon. 2023. PMID: 38261955 Free PMC article.
-
Developments in the Management of Severe Asthma in Children and Adolescents: Focus on Dupilumab and Tezepelumab.Paediatr Drugs. 2023 Nov;25(6):677-693. doi: 10.1007/s40272-023-00589-4. Epub 2023 Sep 2. Paediatr Drugs. 2023. PMID: 37658954 Free PMC article. Review.
-
lncRNA-NEAT1 Sponges miR-128 to Promote Inflammatory Reaction and Phenotypic Transformation of Airway Smooth Muscle Cells.Comput Math Methods Med. 2022 Jan 17;2022:7499911. doi: 10.1155/2022/7499911. eCollection 2022. Comput Math Methods Med. 2022. Retraction in: Comput Math Methods Med. 2023 Jun 28;2023:9782054. doi: 10.1155/2023/9782054. PMID: 35082915 Free PMC article. Retracted.
-
Breathing across ages: a systematic review on challenges and components of transitional care for young people with asthma.Front Pediatr. 2024 Feb 21;12:1348963. doi: 10.3389/fped.2024.1348963. eCollection 2024. Front Pediatr. 2024. PMID: 38450298 Free PMC article. Review.
-
Inhaled corticosteroids in children with persistent asthma: effects on growth.Cochrane Database Syst Rev. 2014 Jul 17;2014(7):CD009471. doi: 10.1002/14651858.CD009471.pub2. Cochrane Database Syst Rev. 2014. PMID: 25030198 Free PMC article.
References
References to studies included in this review
Acun 2005 {published data only}
-
- Acun C, Tomac N, Ermis B, Onk G. Effects of inhaled corticosteroids on growth in asthmatic children: a comparison of fluticasone propionate with budesonide. Allergy and Asthma Proceedings 2005;26(3):204‐6. - PubMed
Ferguson 1999 {published data only}
-
- Ferguson AC, Spier S, Manjra A, Versteegh FG, Mark S, Zhang P. Efficacy and safety of high‐dose inhaled steroids in children with asthma: a comparison of fluticasone propionate with budesonide. Journal of Pediatrics 1999;134(4):422‐7. - PubMed
Ferguson 2007 {published data only}
-
- Ferguson AC, Bever HP, Teper AM, Lasytsya O, Goldfrad CH, Whitehead PJ. A comparison of the relative growth velocities with budesonide and fluticasone propionate in children with asthma. Respiratory Medicine 2007;101(1):118‐29. - PubMed
Pedersen 2002 {published data only}
-
- Pedersen S, Warner J, Wahn U, Staab D, Bourgeois M, Essen‐Zandvliet E, et al. Growth, systemic safety, and efficacy during 1 year of asthma treatment with different beclomethasone dipropionate formulations: an open‐label, randomized comparison of extrafine and conventional aerosols in children. Pediatrics 2002;109(6):e92. - PubMed
Rao 1999 {published data only}
-
- Rao R, Gregson RK, Jones AC, Miles EA, Campbell MJ, Warner JO. Systemic effects of inhaled corticosteroids on growth and bone turnover in childhood asthma: a comparison of fluticasone with beclomethasone. European Respiratory Journal 1999;13(1):87‐94. - PubMed
Vanto 2004 {published data only}
-
- Vanto T, Hämäläinen KM, Vahteristo M, Wille S, Njå F, Hyldebrandt N, et al. Comparison of two budesonide dry powder inhalers in the treatment of asthma in children. Journal of Aerosol Medicine 2004;17(1):15‐24. - PubMed
References to studies excluded from this review
Altintas 2005 {published data only}
-
- Altintas DU, Karakoc GB, Can S, Yilmaz M, Kendirli SG. The effects of long term use of inhaled corticosteroids on linear growth, adrenal function and bone mineral density in children. Allergologia et Immunopathologia 2005;33(4):204‐9. - PubMed
de Benedictis 2000 {published data only}
-
- Benedictis FM, Teper A, Green RJ, Boner AL, Williams L, Medley H, International Study Group. Effects of 2 inhaled corticosteroids on growth: results of a randomized controlled trial. Archives of Pediatrics and Adolescent Medicine 2001;155(11):1248‐54. - PubMed
Delacourt 2003 {published data only}
-
- Delacourt C, Dutau G, Lefrançois G, Clerson P, Beclospin Clinical Development Group. Comparison of the efficacy and safety of nebulized beclometasone dipropionate and budesonide in severe persistent childhood asthma. Respiratory Medicine 2003;97(Suppl B):S27‐33. - PubMed
Ferguson 2001 {published data only}
-
- Ferguson AC. Randomised, double‐blind, double‐dummy, parallel‐group comparison of the effect of fluticasone propionate (FP) 100 μg bd and budesonide (BUD) 200 μg bd on childhood growth: protocol and baseline characteristics. European Respiratory Journal 2001;18:289s.
Ferguson 2002 {published data only}
-
- Ferguson AC. Fluticasone propionate 100 μg bd (FP100) has significantly less effect than budesonide 200 μg bd (BUD200) on childhood growth over 1 year of treatment in asthmatics. European Respiratory Journal 2001;20:219s.
Ferguson 2003 {published data only}
-
- Ferguson AC. Significantly reduced growth velocity over 1 year with budesonide 200 μg bd (BUD200) compared to fluticasone propionate 100 μg bd (FP100) in children with asthma. American Thoracic Society 99th International Conference 2003:A117 Poster D63.
Fitzgerald 1998 {published data only}
Gillman 2002 {published data only}
-
- Gillman SA, Anolik R, Schenkel E, Newman K. One‐year trial on safety and normal linear growth with flunisolide HFA in children with asthma. Clinical Pediatrics 2002;41(5):333‐40. - PubMed
GSK 2005 {published data only}
-
- GSK. A randomised, double‐blind, double‐dummy, parallel group, multicentre study to compare the effect on growth measured by stadiometry of fluticasone propionate (100μg bd) administered via DISKUS™ with budesonide (200μg bd) administered via Turbuhaler in prepubescent children. GSK 2005:study no. FMS40001.
Kannisto 2000 {published data only}
-
- Kannisto S, Korppi M, Remes K, Voutilainen R. Adrenal suppression, evaluated by a low dose adrenocorticotropin test, and growth in asthmatic children treated with inhaled steroids. Journal of Clinical Endocrinology and Metabolism 2000;85(2):652‐7. - PubMed
Kannisto 2002 {published data only}
-
- Kannisto S, Voutilainen R, Remes K, Korppi M. Efficacy and safety of inhaled steroid and cromone treatment in school‐age children: a randomized pragmatic pilot study. Pediatric Allergy and Immunology 2002;13(1):24‐30. - PubMed
Salvatoni 2000 {published data only}
-
- Salvatoni A, Nosetti L, Broggini M, Nespoli L. Body composition and growth in asthmatic children treated with inhaled steroids. Annals of Allergy, Asthma and Immunology 2000;85(3):221‐6. - PubMed
Additional references
Allen 1999
-
- Allen DB. Limitations of short‐term studies in predicting long‐term adverse effects of inhaled corticosteroids. Allergy 1999;54(Suppl 49):29‐34. - PubMed
Allen 2015
-
- Allen DB. Inhaled corticosteroids and growth: still an issue after all these years. Journal of Pediatrics 2015;166(2):463‐9. - PubMed
Asher 2014
-
- Asher I, Pearce N. Global burden of asthma among children. International Journal of Tuberculosis and Lung Disease 2014;18(11):1269‐78. - PubMed
BTS 2016
-
- British Thoracic Society. Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma. A national clinical guideline. www.brit‐thoracic.org.uk/document‐library/clinical‐information/asthma/btssign‐ast... (accessed prior to 28 November 2018).
Colice 2000
-
- Colice GL. Comparing inhaled corticosteroids. Respiratory Care 2000;45(7):846‐53. - PubMed
Djukanovic 1992
-
- Djukanovic R, Wilson JW, Britten KM, Wilson SJ, Walls AF, Roche WR, et al. Effect of an inhaled corticosteroid on airway inflammation and symptoms in asthma. American Review of Respiratory Disease 1992;145(3):669‐74. - PubMed
Efthimiou 1998
-
- Efthimiou J, Barnes PJ. Effect of inhaled corticosteroids on bones and growth. European Respiratory Journal 1998;11(5):1167‐77. - PubMed
Ferrante 2018
FIRS 2017
-
- Forum of International Respiratory Societies. The Global Impact of Respiratory Disease. 2nd Edition. Sheffield: European Respiratory Society, 2017.
Gazala 2005
-
- Gazala E, Sadka R, Bilenko N. Parents’ fears and concerns toward inhaled corticosteroid treatment for their asthmatic children. Pediatric Allergy and Immunology 2005;18(2):82‐7.
GINA 2018
-
- Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention 2018. ginasthma.org/2018‐gina‐report‐global‐strategy‐for‐asthma‐management‐and... (accessed 5 April 2018).
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Kapadia 2016
-
- Kapadia CR, Nebesio TD, Myers SE, Willi S, Miller BS, Allen DB, et al. Endocrine effects of inhaled corticosteroids in children. JAMA Pediatrics 2016;170(2):163‐70. - PubMed
Leung 2017
NHLBI 2007
-
- National Heart, Lung and Blood Institute (NHLBI). Guidelines for the diagnosis and management of asthma (EPR‐3). www.nhlbi.nih.gov/health‐topics/guidelines‐for‐diagnosis‐management‐of‐a... Vol. (accessed 5 April 2018).
NICE 2017
-
- NICE. Asthma: diagnosis, monitoring and chronic asthma management. www.nice.org.uk/guidance/ng80 (accessed prior to 28 November 2018).
Pedersen 2001
-
- Pedersen S. Do inhaled corticosteroids inhibit growth in children?. Americian Journal of Respiratory and Critical CareMedicine 2001;164(4):521‐35. - PubMed
Price 2002
-
- Price J, Hindmarsh P, Hughes S, Efthimiou J. Evaluating the effects of asthma therapy on childhood growth: what can be learnt from the published literature?. European Respiratory Journal 2002;19:1179–93. - PubMed
Pruteanu 2014
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
The Global Asthma Network 2014
-
- Global Asthma Network. The Global Asthma Report 2014. www.globalasthmareport.org/2014/index.php (accessed prior to 28 November 2018).
Wolfgram 2015
Zhang 2011
-
- Zhang L, Axelsson I, Chung M, Lau J. Dose response of inhaled corticosteroids in children with persistent asthma: a systematic review. Pediatrics 2011;127(1):129‐38. - PubMed
Zhang 2014
Zhang 2019
-
- Zhang L, Lasmar LB, Castro‐Rodriguez JA. The impact of asthma and its treatment on growth:an evidence‐based review. Jornal de Pediatria 2019;95(Suppl 1):10‐22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

