Antibiotic therapy for chronic infection with Burkholderia cepacia complex in people with cystic fibrosis
- PMID: 31194880
- PMCID: PMC6564086
- DOI: 10.1002/14651858.CD013079.pub2
Antibiotic therapy for chronic infection with Burkholderia cepacia complex in people with cystic fibrosis
Update in
-
Antibiotic therapy for chronic infection with <I>Burkholderia cepacia</I> complex in people with cystic fibrosis.Cochrane Database Syst Rev. 2021 Dec 10;12(12):CD013079. doi: 10.1002/14651858.CD013079.pub3. Cochrane Database Syst Rev. 2021. PMID: 34889457 Free PMC article.
Abstract
Background: Cystic fibrosis (CF) a life-limiting inherited disease affecting a number of organs, but classically associated with chronic lung infection and progressive loss of lung function. Chronic infection by Burkholderia cepacia complex (BCC) is associated with increased morbidity and mortality and therefore represents a significant challenge to clinicians treating people with CF. This review examines the current evidence for long-term antibiotic therapy in people with CF and chronic BCC infection.
Objectives: The objective of this review is to assess the effects of long-term oral and inhaled antibiotic therapy targeted against chronic BCC lung infections in people with CF. The primary objective is to assess the efficacy of treatments in terms of improvements in lung function and reductions in exacerbation rate. Secondary objectives include quantifying adverse events, mortality and changes in quality of life associated with treatment.
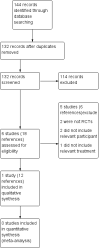
Search methods: We searched the Cochrane Cystic Fibrosis Trials Register, compiled from electronic database searches and handsearching of journals and conference abstract books. We also searched online trial registries and the reference lists of relevant articles and reviews.Date of last search: 29 May 2019.
Selection criteria: Randomised controlled trials (RCTs) of long-term antibiotic therapy in people with CF and chronic BCC infection.
Data collection and analysis: Two authors independently extracted data, assessed risk of bias and assessed the quality of the evidence using GRADE.
Main results: We included one RCT (100 participants) which lasted 52 weeks comparing continuous inhaled aztreonam lysine (AZLI) and placebo in a double-blind RCT for 24 weeks, followed by a 24-week open-label extension and a four-week follow-up period. The average participant age was 26.3 years, 61% were male and average lung function was 56.5% predicted.Treatment with AZLI for 24 weeks was not associated with improvement in forced expiratory volume in one second (FEV1), mean difference 0.91% (95% confidence interval (CI) -3.15 to 4.97) (moderate-quality evidence). The median time to the next exacerbation was 75 days in the AZLI group compared to 51 days in the placebo group, but the difference was not significant (P = 0.27) (moderate-quality evidence). Similarly, the number of participants hospitalised for respiratory exacerbations showed no difference between groups, risk ratio (RR) 0.88 (95% CI 0.53 to 1.45) (moderate-quality evidence). Overall adverse events were similar between groups, RR 1.08 (95% CI 0.98 to 1.19) (moderate-quality evidence). There were no significant differences between treatment groups in relation to mortality (moderate-quality evidence), quality of life or sputum density.In relation to methodological quality, the overall risk of bias in the study was assessed to be unclear to low risk.
Authors' conclusions: We found insufficient evidence from the literature to determine an effective strategy for antibiotic therapy for treating chronic BCC infection.
Conflict of interest statement
FF has received support for educational activities from Chiesi, Gilead Sciences and Vertex. DN has received research grants and support for educational activities from Gilead Sciences.
Figures

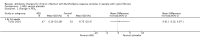
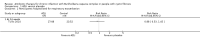


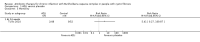
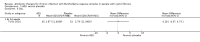
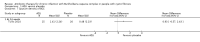
Similar articles
-
Antibiotic treatment for Burkholderia cepacia complex in people with cystic fibrosis experiencing a pulmonary exacerbation.Cochrane Database Syst Rev. 2020 Apr 2;4(4):CD009529. doi: 10.1002/14651858.CD009529.pub4. Cochrane Database Syst Rev. 2020. PMID: 32239690 Free PMC article.
-
Antibiotic therapy for chronic infection with <I>Burkholderia cepacia</I> complex in people with cystic fibrosis.Cochrane Database Syst Rev. 2021 Dec 10;12(12):CD013079. doi: 10.1002/14651858.CD013079.pub3. Cochrane Database Syst Rev. 2021. PMID: 34889457 Free PMC article.
-
Antibiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis.Cochrane Database Syst Rev. 2023 Jun 2;6(6):CD004197. doi: 10.1002/14651858.CD004197.pub6. Cochrane Database Syst Rev. 2023. PMID: 37268599 Free PMC article. Review.
-
Treatments for preventing recurrence of infection with Pseudomonas aeruginosa in people with cystic fibrosis.Cochrane Database Syst Rev. 2019 Dec 17;12(12):CD012300. doi: 10.1002/14651858.CD012300.pub2. Cochrane Database Syst Rev. 2019. PMID: 31845758 Free PMC article.
-
Eradication therapy for Burkholderia cepacia complex in people with cystic fibrosis.Cochrane Database Syst Rev. 2019 Apr 18;4(4):CD009876. doi: 10.1002/14651858.CD009876.pub4. Cochrane Database Syst Rev. 2019. PMID: 30997925 Free PMC article.
Cited by
-
Burkholderia cepacia complex in cystic fibrosis: critical gaps in diagnosis and therapy.Ann Med. 2024 Dec;56(1):2307503. doi: 10.1080/07853890.2024.2307503. Epub 2024 Jan 23. Ann Med. 2024. PMID: 38261606 Free PMC article. Review.
-
Antibiotic treatment for Burkholderia cepacia complex in people with cystic fibrosis experiencing a pulmonary exacerbation.Cochrane Database Syst Rev. 2020 Apr 2;4(4):CD009529. doi: 10.1002/14651858.CD009529.pub4. Cochrane Database Syst Rev. 2020. PMID: 32239690 Free PMC article.
-
Antibiotic Resistance in Patients with Cystic Fibrosis: Past, Present, and Future.Antibiotics (Basel). 2023 Jan 20;12(2):217. doi: 10.3390/antibiotics12020217. Antibiotics (Basel). 2023. PMID: 36830128 Free PMC article. Review.
-
The Impact of Antimicrobial Resistance in Cystic Fibrosis.J Clin Med. 2024 Mar 16;13(6):1711. doi: 10.3390/jcm13061711. J Clin Med. 2024. PMID: 38541936 Free PMC article. Review.
-
[Evidence-based treatment of cystic fibrosis].Internist (Berl). 2020 Dec;61(12):1212-1229. doi: 10.1007/s00108-020-00896-9. Internist (Berl). 2020. PMID: 33201261 Review. German.
References
References to studies included in this review
Tullis 2014 {published data only}
-
- Balfour‐Lynn IM. At last, Burkholderia spp. is one of the inclusion criteria‐‐a negative (but published) randomised controlled trial. Journal of Cystic Fibrosis 2014;13(3):241‐2. [CFGD Register: PI259i] - PubMed
-
- Burns J, LiPuma JJ, Retsch‐Bogart G, Bresnik M, Henig N, McKevitt M, et al. No antibiotic cross‐resistance after 1 year of continuous aztreonam for inhalation solution (AZLI) in cystic fibrosis (CF) patients (pts) with chronic Burkholderia (BURK) infection. Journal of Cystic Fibrosis 2012;11 Suppl 1:S71. [Abstract no.: 58; CFGD Register: PI259d]
-
- Burns JL, LiPuma J, McKevitt M, Lewis S, Retsch‐Bogart GZ, Bresnik M, et al. The effect of burkholderia colony morphology in cystic fibrosis (CF) patients with chronic burkholderia species in a randomized trial of Aztreonam For Inhalation Solution (AZLI). Pediatric Pulmonology 2012;47 Suppl 35:333. [Abstract no.: 307; CFGD Register: PI259g]
-
- EUCTR2009‐011740‐19‐Outside‐EU/EEA. Clinical trial to assess the safety and efficacy of aztreonam for inhalation solution (AZLI) in patients with cystic fibrosis (CF) and chronic burkholderia species infection. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2009‐011740‐19‐Outside‐... (first received 02 February 2015).
-
- NCT01059565. Safety and efficacy study of aztreonam for inhalation solution (AZLI) in patients with cystic fibrosis and chronic burkholderia species infection. https://clinicaltrials.gov/show/nct01059565 (first received 01 February 2010).
References to studies excluded from this review
Adeboyeku 2001 {published data only}
-
- Adeboyeku DU, Agent P, Jackson V, Hodson M. A double blind randomised study to compare the safety and tolerance of differing concentrations of nebulised colistin administered using HaloLite in cystic fibrosis (CF) patients. Pediatric Pulmonology 2001;32 Suppl 22:288. [CFGD Register: PI165]
Burns 1999 {published data only}
-
- Burns JL, Dalfsen JM, Shawar RM, Otto KL, Garber RL, Quan JM, et al. Effect of chronic intermittent administration of inhaled tobramycin on respiratory microbial flora in patients with cystic fibrosis. Journal of Infectious Diseases 1999;179(5):1190‐6. [CFGD Register: PI120i] - PubMed
Kun 1984 {published data only}
-
- Kun P, Landau LI, Phelan PD. Nebulized gentamicin in children and adolescents with cystic fibrosis. Australian Paediatric Journal 1984;20(1):43‐5. [CFGD Register: PI106] - PubMed
Ledson 2002 {published data only}
-
- Ledson MJ, Gallagher MJ, Cowperthwaite C, Robinson M, Convery RP, Walshaw MJ. A randomised double blind placebo controlled crossover trial of nebulised taurolidine in adult CF patients colonised with B Cepacia. 22nd European CF Conference;1998 Jun 13‐19; Berlin. 1998:120. [Abstract no.: PS5‐17]
-
- Ledson MJ, Gallagher MJ, Robinson M, Cowperthwaite C, Williets T, Hart CA, et al. A randomized double‐blinded placebo‐controlled crossover trial of nebulized taurolidine in adult cystic fibrosis patients infected with Burkholderia cepacia. Journal of Aerosol Medicine 2002;15(1):51‐7. [CFGD Register: PI128b] - PubMed
Waters 2017 {published data only}
-
- Waters V, Yau Y, Beaudoin T, Wettlaufer J, Tom SK, McDonald N, et al. Pilot trial of tobramycin inhalation powder in cystic fibrosis patients with chronic Burkholderia cepacia complex infection. Journal of Cystic Fibrosis 2017;16(4):492‐5. - PubMed
Additional references
Aris 2001
-
- Aris RM, Routh JC, LiPuma JJ, Heath DG, Gilligan PH. Lung transplantation for cystic fibrosis patients with Burkholderia cepacia complex. Survival linked to genomovar type. American Journal of Respiratory and Critical Care Medicine 2001;164(11):2102‐6. - PubMed
Bell 2005
-
- Bell SC, Senini SL, McCormack JG. Macrolides in cystic fibrosis. Chronic Respiratory Disease 2005;2(2):85‐98. - PubMed
Burgel 2015
-
- Burgel PR, Bellis G, Olesen HV, Viviani L, Zolin A, Blasi F, et al. Future trends in cystic fibrosis demography in 34 European countries. European Respiratory Journal 2015;46(1):133‐41. - PubMed
CF Trust 2017
-
- Jeffery A, Charman S, Cosgriff R, Carr S. 2016 Annual Data Report. UK CF Registry. London, 2017.
CFF 2016
-
- Cystic Fibrosis Foundation. 2015 Annual Data Report. www.cff.org/Our‐Research/CF‐Patient‐Registry/2015‐Patient‐Registry‐Annua... (accessed 03 April 2018).
Corey 1997
-
- Corey M, Edwards L, Levison H, Knowles M. Longitudinal analysis of pulmonary function decline in patients with cystic fibrosis. Journal of Pediatrics 1997;131(6):809‐14. - PubMed
Drevinek 2010
-
- Drevinek P, Mahenthiralingam E. Burkholderia cenocepacia in cystic fibrosis: epidemiology and molecular mechanisms of virulence. Clinical Microbiology and Infection 2010;16(7):821‐30. - PubMed
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. - PubMed
Farrell 2008
-
- Farrell PM. The prevalence of cystic fibrosis in the European Union. Journal of Cystic Fibrosis 2008;7(5):450‐3. - PubMed
Gibson 2003
-
- Gibson RL, Emerson J, McNamara S, Burns JL, Rosenfeld M, Yunker A, et al. Significant microbiological effect of inhaled tobramycin in young children with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2003;167(6):841‐9. - PubMed
Higgins 2003
Higgins 2011a
-
- Higgins JP, Altman DG, Sterne JA, editor(s) on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
-
- Higgins JP, Deeks JJ, Altman DG, editor(s) on behalf of the Cochrane Statistical Methods Group. Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook of Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Horsley 2016
-
- Horsley A, Jones AM, Lord R. Antibiotic treatment for Burkholderia cepacia complex in people with cystic fibrosis experiencing a pulmonary exacerbation. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, 2016, issue 1. [DOI: 10.1002/14651858.CD009529.pub3; CD009529] - DOI - PMC - PubMed
Jones 2004
Ledson 1998
Lee 2003
-
- Lee TW, Brownlee KG, Conway SP, Denton M, Littlewood JM. Evaluation of a new definition for chronic Pseudomonas aeruginosa infection in cystic fibrosis patients. Journal of Cystic FIbrosis 2003;2(1):29‐34. - PubMed
LiPuma 2010
Mahenthiralingam 2001
-
- Mahenthiralingam E, Vandamme P, Campbell ME, Henry DA, Gravelle AM, Wong LT, et al. Infection with Burkholderia cepacia complex genomovars in patients with cystic fibrosis: virulent transmissible strains of genomovar III can replace Burkholderia multivorans. Clinical Infectious Diseases 2001; Vol. 33, issue 9:1469‐75. - PubMed
Mahenthiralingam 2002
-
- Mahenthiralingam E, Baldwin A, Vandamme P. Burkholderia cepacia complex infection in patients with cystic fibrosis. Journal of Medical Microbiology 2002;51(7):533‐8. - PubMed
McCoy 2008
NICE 2017
-
- NICE. Cystic fibrosis: diagnosis and management. National Institue of Clinical Excellence 2017. - PubMed
Ramsey 1999
-
- Ramsey BW, Pepe MS, Quan JM, Otto KL, Montgomery AB, Williams‐Warren J, et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. New England Journal of Medicine 1999;340(1):23‐30. - PubMed
Regan 2019
Retsch‐Bogart 2008
-
- Retsch‐Bogart GZ, Burns JL, Otto KL, Liou TG, McCoy K, Oermann C, et al. A phase 2 study of aztreonam lysine for inhalation to treat patients with cystic fibrosis and Pseudomonas aeruginosa infection. Pediatric Pulmonology 2008;43(1):47‐58. - PubMed
Ryan 2011
Schünemann 2011b
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al on behalf of the Cochrane Applicability and Recommendations Methods Group and the Cochrane Statistical Methods Group. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Southern 2012
-
- Southern K W. Macrolide antibiotics for cystic fibrosis. Paediatric Respiratory Reviews 2012;13(4):228‐9. - PubMed
Vandamme 2011
-
- Vandamme P, Dawyndt P. Classification and identification of the Burkholderia cepacia complex: past, present and future. Systematic and Applied Microbiology 2011;34(2):87‐95. - PubMed
Wales 1999
Weber 1995
-
- Weber A, Williams‐Warren J, Ramsey B, Smith AL. Tobramycin serum concentrations after aerosol and oral administration in cystic fibrosis. American Journal of Therapeutics 1995;2(2):81‐7. - PubMed
Zlosnik 2015
-
- Zlosnik JE, Zhou G, Brant R, Henry DA, Hird TJ, Mahenthiralingam E, et al. Burkholderia species infections in patients with cystic fibrosis in British Columbia, Canada. 30 years' experience. Annals of the American Thoracic Society 2015;12(1):70‐8. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

