Exposure-Response Analyses of Upadacitinib Efficacy in Phase II Trials in Rheumatoid Arthritis and Basis for Phase III Dose Selection
- PMID: 31194885
- PMCID: PMC6896251
- DOI: 10.1002/cpt.1543
Exposure-Response Analyses of Upadacitinib Efficacy in Phase II Trials in Rheumatoid Arthritis and Basis for Phase III Dose Selection
Abstract
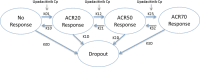
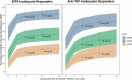
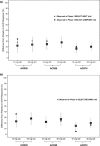
The relationships between upadacitinib, an oral selective Janus kinase 1 inhibitor, plasma exposures, and its efficacy (assessed by the American College of Rheumatology 20%/50%/70% responses over time) in moderate-to-severe active rheumatoid arthritis (RA) were characterized using data from 574 patients, on background methotrexate and inadequate response to methotrexate or anti-TNF therapy, from two phase II trials conducted with twice-daily dosing of an immediate-release formulation. The developed time-continuous Markov models were used to simulate efficacy of once-daily (q.d.). regimens of upadacitinib extended-release incorporating sources of uncertainty. Upadacitinib plasma concentrations associated with 15 and 30 mg extended-release q.d. doses were predicted to achieve that plateau of response across RA subpopulations. Results from these analyses provided the rationale that supported selection and de-risked evaluation of upadacitinib extended-release doses for the first time in >4,000 patients in five large phase III trials.
Trial registration: ClinicalTrials.gov NCT01960855 NCT02066389.
© 2019 AbbVie Inc. Clinical Pharmacology & Therapeutics published by Wiley Periodicals, Inc. on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
M.‐E. Mohamed, B. Klünder, H. S. Camp, and A. A. Othman are employees of AbbVie and may hold AbbVie stock or stock options.
Figures

Similar articles
-
Population Pharmacokinetics of Upadacitinib Using the Immediate-Release and Extended-Release Formulations in Healthy Subjects and Subjects with Rheumatoid Arthritis: Analyses of Phase I-III Clinical Trials.Clin Pharmacokinet. 2019 Aug;58(8):1045-1058. doi: 10.1007/s40262-019-00739-3. Clin Pharmacokinet. 2019. PMID: 30945116 Free PMC article. Clinical Trial.
-
Pharmacokinetics of Upadacitinib With the Clinical Regimens of the Extended-Release Formulation Utilized in Rheumatoid Arthritis Phase 3 Trials.Clin Pharmacol Drug Dev. 2019 Feb;8(2):208-216. doi: 10.1002/cpdd.462. Epub 2018 Apr 24. Clin Pharmacol Drug Dev. 2019. PMID: 29688617 Free PMC article. Clinical Trial.
-
Pharmacokinetics of Upadacitinib in Healthy Subjects and Subjects With Rheumatoid Arthritis, Crohn's Disease, Ulcerative Colitis, or Atopic Dermatitis: Population Analyses of Phase 1 and 2 Clinical Trials.J Clin Pharmacol. 2020 Apr;60(4):528-539. doi: 10.1002/jcph.1550. Epub 2019 Nov 7. J Clin Pharmacol. 2020. PMID: 31701537
-
A review of upadacitinib in rheumatoid arthritis.Mod Rheumatol. 2020 Sep;30(5):779-787. doi: 10.1080/14397595.2020.1782049. Epub 2020 Jul 13. Mod Rheumatol. 2020. PMID: 32530345 Review.
-
Upadacitinib for the treatment of rheumatoid arthritis.Expert Rev Clin Immunol. 2019 Jan;15(1):13-25. doi: 10.1080/1744666X.2019.1544892. Epub 2018 Nov 19. Expert Rev Clin Immunol. 2019. PMID: 30394138 Review.
Cited by
-
Exposure-Response Analyses for Upadacitinib Efficacy in Subjects With Atopic Dermatitis-Analyses of Phase 2b Study to Support Selection of Phase 3 Doses.J Clin Pharmacol. 2021 May;61(5):628-635. doi: 10.1002/jcph.1782. Epub 2020 Dec 5. J Clin Pharmacol. 2021. PMID: 33156550 Free PMC article. Clinical Trial.
-
Pharmacokinetics, Efficacy, and Safety of Upadacitinib in Pediatric Patients With Polyarticular-Course Juvenile Idiopathic Arthritis: An Interim Analysis of an Open-Label, Phase 1 Trial.Arthritis Care Res (Hoboken). 2025 May;77(5):584-593. doi: 10.1002/acr.25465. Epub 2024 Dec 22. Arthritis Care Res (Hoboken). 2025. PMID: 39542836 Free PMC article. Clinical Trial.
-
Effect of Upadacitinib on the Pharmacokinetics of Rosuvastatin or Atorvastatin in Healthy Subjects.Clin Pharmacol Drug Dev. 2021 Nov;10(11):1335-1344. doi: 10.1002/cpdd.957. Epub 2021 Jun 9. Clin Pharmacol Drug Dev. 2021. PMID: 34109764 Free PMC article. Clinical Trial.
-
Exposure-Response Analyses of Upadacitinib Efficacy and Safety in Phase II and III Studies to Support Benefit-Risk Assessment in Rheumatoid Arthritis.Clin Pharmacol Ther. 2020 Apr;107(4):994-1003. doi: 10.1002/cpt.1671. Epub 2019 Nov 30. Clin Pharmacol Ther. 2020. PMID: 31610021 Free PMC article. Clinical Trial.
-
Preferential Inhibition of JAK1 Relative to JAK3 by Upadacitinib: Exposure-Response Analyses of Ex Vivo Data From 2 Phase 1 Clinical Trials and Comparison to Tofacitinib.J Clin Pharmacol. 2020 Feb;60(2):188-197. doi: 10.1002/jcph.1513. Epub 2019 Aug 25. J Clin Pharmacol. 2020. PMID: 31448433 Free PMC article. Clinical Trial.
References
-
- Norman, P. Selective JAK inhibitors in development for rheumatoid arthritis. Expert Opin. Investig. Drugs 23, 1067–1077 (2014). - PubMed
-
- Baker, K.F. & Isaacs, J.D. Novel therapies for immune‐mediated inflammatory diseases: what can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn's disease and ulcerative colitis? Ann. Rheum. Dis. (2017). 10.1136/annrheumdis-2017-211555. - DOI - PubMed
-
- Burmester, G.R. et al Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease‐modifying anti‐rheumatic drugs (SELECT‐NEXT): a randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet 391, 2503–2512 (2018). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

