Exposure-Response Analyses of Upadacitinib Efficacy in Phase II Trials in Rheumatoid Arthritis and Basis for Phase III Dose Selection
- PMID: 31194885
- PMCID: PMC6896251
- DOI: 10.1002/cpt.1543
Exposure-Response Analyses of Upadacitinib Efficacy in Phase II Trials in Rheumatoid Arthritis and Basis for Phase III Dose Selection
Abstract
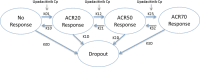
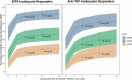
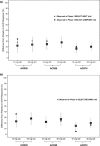
The relationships between upadacitinib, an oral selective Janus kinase 1 inhibitor, plasma exposures, and its efficacy (assessed by the American College of Rheumatology 20%/50%/70% responses over time) in moderate-to-severe active rheumatoid arthritis (RA) were characterized using data from 574 patients, on background methotrexate and inadequate response to methotrexate or anti-TNF therapy, from two phase II trials conducted with twice-daily dosing of an immediate-release formulation. The developed time-continuous Markov models were used to simulate efficacy of once-daily (q.d.). regimens of upadacitinib extended-release incorporating sources of uncertainty. Upadacitinib plasma concentrations associated with 15 and 30 mg extended-release q.d. doses were predicted to achieve that plateau of response across RA subpopulations. Results from these analyses provided the rationale that supported selection and de-risked evaluation of upadacitinib extended-release doses for the first time in >4,000 patients in five large phase III trials.
Trial registration: ClinicalTrials.gov NCT01960855 NCT02066389.
© 2019 AbbVie Inc. Clinical Pharmacology & Therapeutics published by Wiley Periodicals, Inc. on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
M.‐E. Mohamed, B. Klünder, H. S. Camp, and A. A. Othman are employees of AbbVie and may hold AbbVie stock or stock options.
Figures

References
-
- Norman, P. Selective JAK inhibitors in development for rheumatoid arthritis. Expert Opin. Investig. Drugs 23, 1067–1077 (2014). - PubMed
-
- Baker, K.F. & Isaacs, J.D. Novel therapies for immune‐mediated inflammatory diseases: what can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn's disease and ulcerative colitis? Ann. Rheum. Dis. (2017). 10.1136/annrheumdis-2017-211555. - DOI - PubMed
-
- Burmester, G.R. et al Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease‐modifying anti‐rheumatic drugs (SELECT‐NEXT): a randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet 391, 2503–2512 (2018). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

