Apolipoprotein E and Periostin Are Potential Biomarkers of Nasal Mucosal Inflammation. A Parallel Approach of In Vitro and In Vivo Secretomes
- PMID: 31194918
- PMCID: PMC7051477
- DOI: 10.1165/rcmb.2018-0248OC
Apolipoprotein E and Periostin Are Potential Biomarkers of Nasal Mucosal Inflammation. A Parallel Approach of In Vitro and In Vivo Secretomes
Abstract
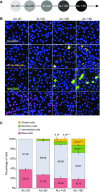
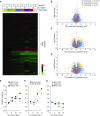
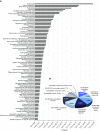
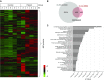
No previously suggested biomarkers of nasal mucosal inflammation have been practically applied in clinical fields, and nasal epithelium-derived secreted proteins as biomarkers have not specifically been investigated. The goal of this study was to identify secreted proteins that dynamically change during the differentiation from basal cells to fully differentiated cells and examine whether nasal epithelium-derived proteins can be used as biomarkers of nasal mucosal inflammation, such as chronic rhinosinusitis. To achieve this goal, we analyzed two secretomes using the isobaric tag for relative and absolute quantification technique. From in vitro secretomes, we identified the proteins altered in apical secretions of primary human nasal epithelial cells according to the degree of differentiation; from in vivo secretomes, we identified the increased proteins in nasal lavage fluids obtained from patients 2 weeks after endoscopic sinus surgery for chronic sinusitis. We then used a parallel approach to identify specific biomarkers of nasal mucosal inflammation; first, we selected apolipoprotein E as a nasal epithelial cell-derived biomarker through screening proteins that were upregulated in both in vitro and in vivo secretomes, and verified highly secreted apolipoprotein E in nasal lavage fluids of the patients by Western blotting. Next, we selected periostin as an inflammatory mediator-inducible biomarker from in vivo secretomes, the secretion of which was not induced under in vitro culture conditions. We demonstrated that those two nasal epithelium-derived proteins are possible biomarkers of nasal mucosal inflammation.
Keywords: air–liquid interface culture; mucosal secretions; nasal epithelium; secreted proteins; secretome.
Figures


Similar articles
-
Oncostatin M promotes mucosal epithelial barrier dysfunction, and its expression is increased in patients with eosinophilic mucosal disease.J Allergy Clin Immunol. 2015 Sep;136(3):737-746.e4. doi: 10.1016/j.jaci.2015.01.043. Epub 2015 Apr 1. J Allergy Clin Immunol. 2015. PMID: 25840724 Free PMC article.
-
Expression of pendrin and periostin in allergic rhinitis and chronic rhinosinusitis.Allergol Int. 2012 Dec;61(4):589-95. doi: 10.2332/allergolint.11-OA-0370. Epub 2012 Aug 25. Allergol Int. 2012. PMID: 22918213
-
Differential expression of periostin in the nasal polyp may represent distinct histological features of chronic rhinosinusitis.Auris Nasus Larynx. 2015 Apr;42(2):123-7. doi: 10.1016/j.anl.2014.09.003. Epub 2014 Sep 27. Auris Nasus Larynx. 2015. PMID: 25270863
-
Epithelial-Cell-Derived Extracellular Vesicles in Pathophysiology of Epithelial Injury and Repair in Chronic Rhinosinusitis: Connecting Immunology in Research Lab to Biomarkers in Clinics.Int J Mol Sci. 2021 Oct 28;22(21):11709. doi: 10.3390/ijms222111709. Int J Mol Sci. 2021. PMID: 34769139 Free PMC article. Review.
-
A Comprehensive Systematic Review of the Association Between Airway Mucins and Chronic Rhinosinusitis.Am J Rhinol Allergy. 2019 Jul;33(4):433-448. doi: 10.1177/1945892419837042. Epub 2019 Mar 20. Am J Rhinol Allergy. 2019. PMID: 30892914 No abstract available.
Cited by
-
High-Density Lipoprotein (HDL) in Allergy and Skin Diseases: Focus on Immunomodulating Functions.Biomedicines. 2020 Dec 1;8(12):558. doi: 10.3390/biomedicines8120558. Biomedicines. 2020. PMID: 33271807 Free PMC article. Review.
-
Airway epithelial CD47 plays a critical role in inducing influenza virus-mediated bacterial super-infection.Nat Commun. 2024 Apr 30;15(1):3666. doi: 10.1038/s41467-024-47963-5. Nat Commun. 2024. PMID: 38693120 Free PMC article.
-
Recent advances in mass spectrometry-based proteomics and metabolomics in chronic rhinosinusitis with nasal polyps.Front Immunol. 2023 Sep 6;14:1267194. doi: 10.3389/fimmu.2023.1267194. eCollection 2023. Front Immunol. 2023. PMID: 37744372 Free PMC article. Review.
-
Caesalpinia sappan Linn. Ameliorates Allergic Nasal Inflammation by Upregulating the Keap1/Nrf2/HO-1 Pathway in an Allergic Rhinitis Mouse Model and Nasal Epithelial Cells.Antioxidants (Basel). 2022 Nov 15;11(11):2256. doi: 10.3390/antiox11112256. Antioxidants (Basel). 2022. PMID: 36421442 Free PMC article.
-
The Multiple Roles of Periostin in Non-Neoplastic Disease.Cells. 2022 Dec 22;12(1):50. doi: 10.3390/cells12010050. Cells. 2022. PMID: 36611844 Free PMC article. Review.
References
-
- Rogers DF. Airway goblet cells: responsive and adaptable front-line defenders. Eur Respir J. 1994;7:1690–1706. - PubMed
-
- Mercer RR, Russell ML, Roggli VL, Crapo JD. Cell number and distribution in human and rat airways. Am J Respir Cell Mol Biol. 1994;10:613–624. - PubMed
-
- Boers JE, Ambergen AW, Thunnissen FB. Number and proliferation of basal and parabasal cells in normal human airway epithelium. Am J Respir Crit Care Med. 1998;157:2000–2006. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

