An acyl-CoA dehydrogenase microplate activity assay using recombinant porcine electron transfer flavoprotein
- PMID: 31194945
- PMCID: PMC6661201
- DOI: 10.1016/j.ab.2019.06.003
An acyl-CoA dehydrogenase microplate activity assay using recombinant porcine electron transfer flavoprotein
Abstract
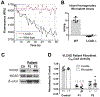
Acyl-CoA dehydrogenases (ACADs) play key roles in the mitochondrial catabolism of fatty acids and branched-chain amino acids. All nine characterized ACAD enzymes use electron transfer flavoprotein (ETF) as their redox partner. The gold standard for measuring ACAD activity is the anaerobic ETF fluorescence reduction assay, which follows the decrease of pig ETF fluorescence as it accepts electrons from an ACAD in vitro. Although first described 35 years ago, the assay has not been widely used due to the need to maintain an anaerobic assay environment and to purify ETF from pig liver mitochondria. Here, we present a method for expressing recombinant pig ETF in E coli and purifying it to homogeneity. The recombinant protein is virtually pure after one chromatography step, bears higher intrinsic fluorescence than the native enzyme, and provides enhanced activity in the ETF fluorescence reduction assay. Finally, we present a simplified protocol for removing molecular oxygen that allows adaption of the assay to a 96-well plate format. The availability of recombinant pig ETF and the microplate version of the ACAD activity assay will allow wide application of the assay for both basic research and clinical diagnostics.
Keywords: Acyl-CoA dehydrogenase; Electron transfer flavoprotein; Enzyme activity assay; Fatty acid oxidation; Mitochondria.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures
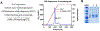

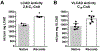


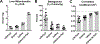
References
-
- Houten SM, Violante S, Ventura FV, and Wanders RJ (2016) The Biochemistry and Physiology of Mitochondrial Fatty Acid beta-Oxidation and Its Genetic Disorders. Annual review of physiology 78, 23–44 - PubMed
-
- Repp BM, Mastantuono E, Alston CL, Schiff M, Haack TB, Rotig A, Ardissone A, Lombes A, Catarino CB, Diodato D, Schottmann G, Poulton J, Burlina A, Jonckheere A, Munnich A, Rolinski B, Ghezzi D, Rokicki D, Wellesley D, Martinelli D, Wenhong D, Lamantea E, Ostergaard E, Pronicka E, Pierre G, Smeets HJM, Wittig I, Scurr I, de Coo IFM, Moroni I, Smet J, Mayr JA, Dai L, de Meirleir L, Schuelke M, Zeviani M, Morscher RJ, McFarland R, Seneca S, Klopstock T, Meitinger T, Wieland T, Strom TM, Herberg U, Ahting U, Sperl W, Nassogne MC, Ling H, Fang F, Freisinger P, Van Coster R, Strecker V, Taylor RW, Haberle J, Vockley J, Prokisch H, and Wortmann S (2018) Clinical, biochemical and genetic spectrum of 70 patients with ACAD9 deficiency: is riboflavin supplementation effective? Orphanet journal of rare diseases 13, 120. - PMC - PubMed
-
- Rennison JH, McElfresh TA, Okere IC, Patel HV, Foster AB, Patel KK, Stoll MS, Minkler PE, Fujioka H, Hoit BD, Young ME, Hoppel CL, and Chandler MP (2008) Enhanced acyl-CoA dehydrogenase activity is associated with improved mitochondrial and contractile function in heart failure. Cardiovascular research 79, 331–340 - PubMed
-
- Goetzman ES (2009) The regulation of acyl-CoA dehydrogenases in adipose tissue by rosiglitazone. Obesity 17, 196–198 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

