Genetic toxicology in silico protocol
- PMID: 31195068
- PMCID: PMC7485926
- DOI: 10.1016/j.yrtph.2019.104403
Genetic toxicology in silico protocol
Abstract
In silico toxicology (IST) approaches to rapidly assess chemical hazard, and usage of such methods is increasing in all applications but especially for regulatory submissions, such as for assessing chemicals under REACH as well as the ICH M7 guideline for drug impurities. There are a number of obstacles to performing an IST assessment, including uncertainty in how such an assessment and associated expert review should be performed or what is fit for purpose, as well as a lack of confidence that the results will be accepted by colleagues, collaborators and regulatory authorities. To address this, a project to develop a series of IST protocols for different hazard endpoints has been initiated and this paper describes the genetic toxicity in silico (GIST) protocol. The protocol outlines a hazard assessment framework including key effects/mechanisms and their relationships to endpoints such as gene mutation and clastogenicity. IST models and data are reviewed that support the assessment of these effects/mechanisms along with defined approaches for combining the information and evaluating the confidence in the assessment. This protocol has been developed through a consortium of toxicologists, computational scientists, and regulatory scientists across several industries to support the implementation and acceptance of in silico approaches.
Keywords: (Q)SAR; Computational toxicology protocols; Expert alerts; Expert review; Genetic toxicology; In silico; In silico toxicology.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
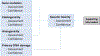

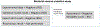
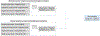

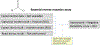



References
-
- CHEMOINFORMATICS AND BEYOND. Chemoinformatics for Drug Discovery.
-
- Ahlberg E, Amberg A, Beilke LD, Bower D, Cross KP, Custer L, Ford KA, Van Gompel J, Harvey J, Honma M, Jolly R, Joossens E, Kemper RA, Kenyon M, Kruhlak N, Kuhnke L, Leavitt P, Naven R, Neilan C, Quigley DP, Shuey D, Spirkl HP, Stavitskaya L, Teasdale A, White A, Wichard J, Zwickl C, Myatt GJ, 2016. Extending (Q)SARs to incorporate proprietary knowledge for regulatory purposes: A case study using aromatic amine mutagenicity. Regul Toxicol Pharmacol 77, 1–12. 10.1016/j.yrtph.2016.02.003 - DOI - PubMed
-
- Amberg A, Beilke L, Bercu J, Bower D, Brigo A, Cross KP, Custer L, Dobo K, Dowdy E, Ford KA, Glowienke S, Van Gompel J, Harvey J, Hasselgren C, Honma M, Jolly R, Kemper R, Kenyon M, Kruhlak N, Leavitt P, Miller S, Muster W, Nicolette J, Plaper A, Powley M, Quigley DP, Reddy MV, Spirkl HP, Stavitskaya L, Teasdale A, Weiner S, Welch DS, White A, Wichard J, Myatt GJ, 2016. Principles and procedures for implementation of ICH M7 recommended (Q)SAR analyses. Regul Toxicol Pharmacol 77, 13–24. 10.1016/j.yrtph.2016.02.004 - DOI - PubMed
-
- Amberg A, Harvey JS, Czich A, Spirkl H-P, Robinson S, White A, Elder DP, 2015. Do Carboxylic/Sulfonic Acid Halides Really Present a Mutagenic and Carcinogenic Risk as Impurities in Final Drug Products? Organic Process Research & Development 19, 1495–1506. 10.1021/acs.oprd.5b00106 - DOI
-
- Ashby J, Tennant RW, 1988. Chemical structure, Salmonella mutagenicity and extent of carcinogenicity as indicators of genotoxic carcinogenesis among 222 chemicals tested in rodents by the U.S. NCI/NTP. Mutat Res 204, 17–115. - PubMed

