Safety and efficacy of first-line smoking cessation pharmacotherapies in bipolar disorders: Subgroup analysis of a randomized clinical trial
- PMID: 31195244
- PMCID: PMC8936081
- DOI: 10.1016/j.jad.2019.06.008
Safety and efficacy of first-line smoking cessation pharmacotherapies in bipolar disorders: Subgroup analysis of a randomized clinical trial
Abstract
Objectives: Post hoc analyses of EAGLES data to examine safety and efficacy of first-line smoking cessation pharmacotherapies in smokers with bipolar disorders (BD).
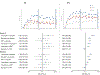
Methods: Smokers with BD I/II (n = 285; 81.4% with BD I) and a comparison nonpsychiatric cohort (NPC; n = 2794) were randomly assigned to varenicline, bupropion, nicotine replacement therapy (NRT), or placebo for 12 weeks, plus weekly counseling. Primary outcomes were occurrence of moderate to severe neuropsychiatric adverse events (NPSAEs) and Weeks 9-12 biochemically-confirmed continuous abstinence (CA) rates.
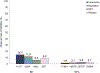
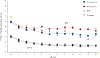
Results: For BD smokers, NPSAE risk differences versus placebo were: varenicline, 6.17 (95% CI: -7.84 to 20.18); bupropion, 4.09 (-8.82 to 16.99); NRT, -0.56 (-12.34 to 11.22). ORs for Weeks 9-12 CA, comparing active medication to placebo among BD smokers were: varenicline, 2.61 (0.68-9.95); bupropion, 1.29 (0.31-5.37), NRT, 0.71 (0.14-3.74). Pooling across treatments, NPSAE occurrence was higher (10.7% versus 2.3%; P < 0.001) and CA rates were lower (22.8% versus 13.3%; P = 0.008) in BD than NPC.
Limitations: Study not powered to detect differences in safety and efficacy in the BD subcohort; generalizability limited to stably treated BD without current substance use disorders.
Conclusions: Smokers with BD had higher risk of NPSAEs and were less likely to quit overall than NPC smokers. Among smokers with BD, NPSAE risk difference estimates for active treatments versus placebo ranged from 1% lower to 6% higher. Efficacy of varenicline in smokers with BD was similar to EAGLES main outcomes; bupropion and NRT effect sizes were descriptively lower. Varenicline may be a tolerable and effective cessation treatment for smokers with BD.
Trial registration: ClinicalTrials.gov identifier (https://clinicaltrials.gov/): NCT01456936.
Keywords: Abbreviations: BD, bipolar disorders; Bipolar disorder; CA, continuous abstinence; CAR, continuous abstinence rate; Efficacy; NPC, nonpsychiatric cohort; NPSAE, neuropsychiatric adverse event; PPA, point prevalence abstinence; Safety; Smoking cessation; Varenicline.
Copyright © 2019. Published by Elsevier B.V.
Figures


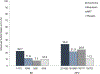
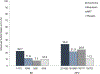
References
-
- American Psychiatric Association, 2000. Diagnostic and Statistical Manual of Mental Disorders, 4th ed. American Psychiatric Association, Arlington, VA.
-
- Anthenelli RM, Benowitz NL, West R, St Aubin L, McRae T, Lawrence D, Ascher J, Russ C, Krishen A, Evins AE, 2016. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet 387, 2507–2520. - PubMed
-
- Benowitz NL, Jacob III P, Ahijevych K, Jarvis MJ, Hall S, LeHouezec J, Hansson A, Lichtenstein E, Henningfield J, Tsoh J, Hurt RD, Velicer W, (SRNT Subcommittee on Biochemical Verification), 2002. Biochemical verification of tobacco use and cessation. Nicotine Tob Res 4, 149–159. - PubMed
-
- Breslau N, Kilbey MM, Andreski P, 1992. Nicotine withdrawal symptoms and psychiatric disorders: findings from an epidemiologic study of young adults. Am J Psychiatry 149, 464–469. - PubMed
-
- Brunette MF, Pratt SI, Bartels SJ, Scherer EA, Sigmon SC, Ferron JC, Santos M, Williams GE, Kosydar S, Wolfe RS, Lotz D, Capuchino K, 2018. Randomized trial of interventions for smoking cessation among Medicaid beneficiaries with mental illness. Psychiatr Serv 69, 274–280. - PubMed

