An NIR emitting styryl dye with large Stokes shift to enable co-staining study on zebrafish neuromast hair cells
- PMID: 31195328
- PMCID: PMC6656593
- DOI: 10.1016/j.bioorg.2019.103040
An NIR emitting styryl dye with large Stokes shift to enable co-staining study on zebrafish neuromast hair cells
Abstract
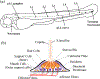
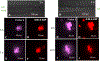
Hearing loss is a significant public health problem, and the "loss of sensory hair cells" is one of two leading causes in humans. Advanced imaging reagents are desirable for understanding the role of the surrounding support cells in the loss or regeneration of the hair cells. A styryl dye was found to exhibit NIR emission (λem ≈ 684 nm) with a very large Stokes shift (Δν ≈ 9190 cm-1), due to the incorporation of excited state intramolecular proton transfer (ESIPT) mechanism. When used to stain live zebrafish embryos, the probe was found to exhibit good selectivity in targeting neuromasts, which are sensory organs on the surface of the fish's body. The finding was verified by direct comparison with the known neuromast-labeling reagent, 4-Di-2-ASP. In contrast to the existing styryl dyes that label neuromast hair cells, the new probe labeled both neuromast hair cells and the surrounding support cells, while giving discernable signals. The study thus illustrated a useful tool to aid the developmental study of two closely related cell types on the mechanosensory sensory organ of zebrafish, which is a powerful animal model for hearing loss research.
Keywords: Cyanine; Excited state intramolecular proton transfer; Near Infrared (NIR) emission; Neuromasts; Styryl dye; Support cell; Zebrafish.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures
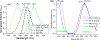
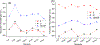



Similar articles
-
NIR-emitting styryl dyes with large Stokes' shifts for imaging application: From cellular plasma membrane, mitochondria to Zebrafish neuromast.Dyes Pigm. 2021 Oct;194:109629. doi: 10.1016/j.dyepig.2021.109629. Epub 2021 Jul 6. Dyes Pigm. 2021. PMID: 34366501 Free PMC article.
-
A NIR-emitting cyanine with large Stokes shifts for live cell imaging: large impact of the phenol group on emission.Chem Commun (Camb). 2019 Oct 31;55(88):13223-13226. doi: 10.1039/c9cc06831g. Chem Commun (Camb). 2019. PMID: 31595909 Free PMC article.
-
Labeling hair cells and afferent neurons in the posterior lateral-line system of zebrafish.Cold Spring Harb Protoc. 2013 Dec 1;2013(12):1172-4. doi: 10.1101/pdb.prot079467. Cold Spring Harb Protoc. 2013. PMID: 24298034
-
Zebrafish (Danio rerio) neuromast: promising biological endpoint linking developmental and toxicological studies.Aquat Toxicol. 2009 Dec 13;95(4):307-19. doi: 10.1016/j.aquatox.2009.04.007. Epub 2009 Apr 24. Aquat Toxicol. 2009. PMID: 19467721 Review.
-
Zebrafish neuromast sensory system: Is it an emerging target to assess environmental pollution impacts?Environ Pollut. 2024 Mar 1;344:123400. doi: 10.1016/j.envpol.2024.123400. Epub 2024 Jan 23. Environ Pollut. 2024. PMID: 38272167 Review.
Cited by
-
Progress in Tuning Emission of the Excited-State Intramolecular Proton Transfer (ESIPT)-Based Fluorescent Probes.ACS Omega. 2021 Mar 4;6(10):6547-6553. doi: 10.1021/acsomega.0c06252. eCollection 2021 Mar 16. ACS Omega. 2021. PMID: 33748566 Free PMC article. Review.
-
Fluorescence Lifetimes of NIR-Emitting Molecules with Excited-State Intramolecular Proton Transfer.Molecules. 2022 Dec 23;28(1):125. doi: 10.3390/molecules28010125. Molecules. 2022. PMID: 36615319 Free PMC article.
-
NIR-emitting styryl dyes with large Stokes' shifts for imaging application: From cellular plasma membrane, mitochondria to Zebrafish neuromast.Dyes Pigm. 2021 Oct;194:109629. doi: 10.1016/j.dyepig.2021.109629. Epub 2021 Jul 6. Dyes Pigm. 2021. PMID: 34366501 Free PMC article.
-
Cationic styryl dyes for DNA labelling and selectivity toward cancer cells and Gram-negative bacteria.RSC Adv. 2023 Jan 11;13(3):2115-2122. doi: 10.1039/d2ra07601b. eCollection 2023 Jan 6. RSC Adv. 2023. PMID: 36712646 Free PMC article.
-
Design and Synthesis of ESIPT-Based Imidazole Derivatives for Cell Imaging.ACS Omega. 2024 May 27;9(23):24291-24298. doi: 10.1021/acsomega.3c09822. eCollection 2024 Jun 11. ACS Omega. 2024. PMID: 38882084 Free PMC article.
References
-
- Froehlicher M, Liedtke A, Groh KJ, Neuhauss SCF, Segner H, Eggen RIL, Zebrafish (Danio rerio) neuromast: Promising biological endpoint linking developmental and toxicological studies, Aquatic Toxicology. 95 (4) (2009) 307–319. - PubMed
-
- Nechiporuk A, Raible DW, FGF-Dependent Mechanosensory Organ Patterning in Zebrafish, Science. 320 (5884) (2008), 1774. - PubMed
-
- Ghysen A, Dambly-Chaudiere C, Development of the zebrafish lateral line, Current Opinion in Neurobiology. 14 (1) (2004), 67–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

