Mitochondrial Activity and Skeletal Muscle Insulin Resistance in Kidney Disease
- PMID: 31195596
- PMCID: PMC6600571
- DOI: 10.3390/ijms20112751
Mitochondrial Activity and Skeletal Muscle Insulin Resistance in Kidney Disease
Abstract
Insulin resistance is a key feature of the metabolic syndrome, a cluster of medical disorders that together increase the chance of developing type 2 diabetes and cardiovascular disease. In turn, type 2 diabetes may cause complications such as diabetic kidney disease (DKD). Obesity is a major risk factor for developing systemic insulin resistance, and skeletal muscle is the first tissue in susceptible individuals to lose its insulin responsiveness. Interestingly, lean individuals are not immune to insulin resistance either. Non-obese, non-diabetic subjects with chronic kidney disease (CKD), for example, exhibit insulin resistance at the very onset of CKD, even before clinical symptoms of renal failure are clear. This uraemic insulin resistance contributes to the muscle weakness and muscle wasting that many CKD patients face, especially during the later stages of the disease. Bioenergetic failure has been associated with the loss of skeletal muscle insulin sensitivity in obesity and uraemia, as well as in the development of kidney disease and its sarcopenic complications. In this mini review, we evaluate how mitochondrial activity of different renal cell types changes during DKD progression, and discuss the controversial role of oxidative stress and mitochondrial reactive oxygen species in DKD. We also compare the involvement of skeletal muscle mitochondria in uraemic and obesity-related muscle insulin resistance.
Keywords: ATP turnover; bioenergetics; diabetic nephropathy; energy metabolism; insulin signalling; muscle wasting; obesity; oxidative stress; renal sarcopenia; uraemic myopathy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
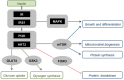
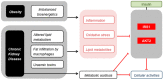
Similar articles
-
Mitochondrial involvement in skeletal muscle insulin resistance: A case of imbalanced bioenergetics.Biochim Biophys Acta. 2016 Oct;1857(10):1678-93. doi: 10.1016/j.bbabio.2016.07.008. Epub 2016 Jul 26. Biochim Biophys Acta. 2016. PMID: 27473535 Review.
-
Unacylated ghrelin normalizes skeletal muscle oxidative stress and prevents muscle catabolism by enhancing tissue mitophagy in experimental chronic kidney disease.FASEB J. 2017 Dec;31(12):5159-5171. doi: 10.1096/fj.201700126R. Epub 2017 Aug 4. FASEB J. 2017. PMID: 28778977
-
The manifold role of the mitochondria in skeletal muscle insulin resistance.Curr Opin Clin Nutr Metab Care. 2018 Jul;21(4):267-272. doi: 10.1097/MCO.0000000000000480. Curr Opin Clin Nutr Metab Care. 2018. PMID: 29847447 Free PMC article. Review.
-
Mitochondrial biogenesis: pharmacological approaches.Curr Pharm Des. 2014;20(35):5507-9. doi: 10.2174/138161282035140911142118. Curr Pharm Des. 2014. PMID: 24606795
-
Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle.Mol Cell Biol. 2012 Jan;32(2):309-19. doi: 10.1128/MCB.05603-11. Epub 2011 Nov 14. Mol Cell Biol. 2012. PMID: 22083962 Free PMC article.
Cited by
-
Mechanism of Tripterygium wilfordii Hook.F.- Trichosanthes kirilowii Maxim decoction in treatment of diabetic kidney disease based on network pharmacology and molecular docking.Front Pharmacol. 2022 Oct 28;13:940773. doi: 10.3389/fphar.2022.940773. eCollection 2022. Front Pharmacol. 2022. PMID: 36386135 Free PMC article.
-
Bed-side measures for diagnosis of low muscle mass, sarcopenia, obesity, and sarcopenic obesity in patients with chronic kidney disease under non-dialysis-dependent, dialysis dependent and kidney transplant therapy.PLoS One. 2020 Nov 20;15(11):e0242671. doi: 10.1371/journal.pone.0242671. eCollection 2020. PLoS One. 2020. PMID: 33216775 Free PMC article.
-
Brain-derived neurotrophic factor associated with kidney function.Diabetol Metab Syndr. 2023 Feb 13;15(1):16. doi: 10.1186/s13098-023-00991-5. Diabetol Metab Syndr. 2023. PMID: 36782254 Free PMC article.
-
Muscle Stem Cell-Derived Extracellular Vesicles Reverse Hydrogen Peroxide-Induced Mitochondrial Dysfunction in Mouse Myotubes.Cells. 2020 Nov 26;9(12):2544. doi: 10.3390/cells9122544. Cells. 2020. PMID: 33256005 Free PMC article.
-
TLR13 contributes to skeletal muscle atrophy by increasing insulin resistance in chronic kidney disease.Cell Prolif. 2022 Mar;55(3):e13181. doi: 10.1111/cpr.13181. Epub 2022 Jan 28. Cell Prolif. 2022. PMID: 35088922 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

