Design, Synthesis and Antibacterial Activity of Coumarin-1,2,3-triazole Hybrids Obtained from Natural Furocoumarin Peucedanin
- PMID: 31195697
- PMCID: PMC6600338
- DOI: 10.3390/molecules24112126
Design, Synthesis and Antibacterial Activity of Coumarin-1,2,3-triazole Hybrids Obtained from Natural Furocoumarin Peucedanin
Abstract
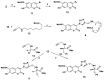
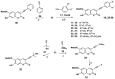
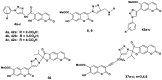
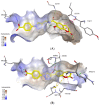
Synthesis of 1,2,3-triazole-substituted coumarins and also 1,2,3-triazolyl or 1,2,3-triazolylalk-1-inyl-linked coumarin-2,3-furocoumarin hybrids was performed by employing the cross-coupling and copper catalyzed azide-alkyne cycloaddition reaction approaches. The synthesized compounds were evaluated for their in vitro antibacterial activity against Staphylococcus aureus, Bacillius subtilis, Actinomyces viscosus and Escherichia coli bacterial strains. Coumarin-benzoic acid hybrids 4с, 42с and 3-((4-acetylamino-3-(methoxycarbonyl)phenyl)ethynyl)coumarin (29) showed promising activity against S. aureus strains, and the 1,2,3-triazolyloct-1-inyl linked coumarin-2,3-furocoumarin hybrid 37c was endowed with high selectivity against B. subtilis and E. coli species. The in vitro antibacterial activity of 4с, 29, 37c and 42с can potentially be compared with that of a number of modern antibiotic drugs used in the clinic, suggesting promising prospects for further research. A detailed study of the molecular interactions with the targeted protein MurB was performed using docking simulations and the obtained results are quite promising.
Keywords: CuAAC reaction; Sonogashira coupling; antibacterial activity; coumarin; furocoumarin.
Conflict of interest statement
The authors declare that they have no conflict of interests.
Figures
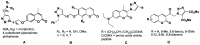



References
-
- Ashok D., Gundu S., Aamate V.K., Devulapally M.G., Bathini R., Manga V. Dimers of coumarin-1,2,3-triazole hybrids bearing alkyl spacer: Design, microwave-assisted synthesis, molecular docking andevaluation as antimycobacterial and antimicrobial agents. J. Mol. Sructure. 2018;1157:312–321. doi: 10.1016/j.molstruc.2017.12.080. - DOI
-
- Chougala B.M., Samundeeswari S., Holiyachi M., Shastri L.A., Dodamani S., Jalapure S., Dixit S.R., Joshi S.D., Sunagar V.A. Synthesis, characterization and molecular docking studies of substituted 4-coumarinylpyrano[2,3-c]pyrazole derivatives as potent antibacterial and anti-inflammatory agents. Eur. J. Med. Chem. 2017;125:101–116. doi: 10.1016/j.ejmech.2016.09.021. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

