Towards CMOS Integrated Microfluidics Using Dielectrophoretic Immobilization
- PMID: 31195725
- PMCID: PMC6628019
- DOI: 10.3390/bios9020077
Towards CMOS Integrated Microfluidics Using Dielectrophoretic Immobilization
Abstract
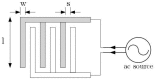
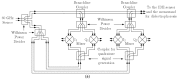
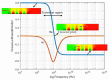
Dielectrophoresis (DEP) is a nondestructive and noninvasive method which is favorable for point-of-care medical diagnostic tests. This technique exhibits prominent relevance in a wide range of medical applications wherein the miniaturized platform for manipulation (immobilization, separation or rotation), and detection of biological particles (cells or molecules) can be conducted. DEP can be performed using advanced planar technologies, such as complementary metal-oxide-semiconductor (CMOS) through interdigitated capacitive biosensors. The dielectrophoretically immobilization of micron and submicron size particles using interdigitated electrode (IDE) arrays is studied by finite element simulations. The CMOS compatible IDEs have been placed into the silicon microfluidic channel. A rigorous study of the DEP force actuation, the IDE's geometrical structure, and the fluid dynamics are crucial for enabling the complete platform for CMOS integrated microfluidics and detection of micron and submicron-sized particle ranges. The design of the IDEs is performed by robust finite element analyses to avoid time-consuming and costly fabrication processes. To analyze the preliminary microfluidic test vehicle, simulations were first performed with non-biological particles. To produce DEP force, an AC field in the range of 1 to 5 V (peak-to-peak) is applied to the IDE. The impact of the effective external and internal properties, such as actuating DEP frequency and voltage, fluid flow velocity, and IDE's geometrical parameters are investigated. The IDE based system will be used to immobilize and sense particles simultaneously while flowing through the microfluidic channel. The sensed particles will be detected using the capacitive sensing feature of the biosensor. The sensing and detecting of the particles are not in the scope of this paper and will be described in details elsewhere. However, to provide a complete overview of this system, the working principles of the sensor, the readout detection circuit, and the integration process of the silicon microfluidic channel are briefly discussed.
Keywords: CMOS biosensor; biomolecules; dielectrophoretic immobilization; lab-on-chip; microfluidic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

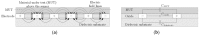










Similar articles
-
Characterization and Separation of Live and Dead Yeast Cells Using CMOS-Based DEP Microfluidics.Micromachines (Basel). 2021 Mar 6;12(3):270. doi: 10.3390/mi12030270. Micromachines (Basel). 2021. PMID: 33800809 Free PMC article.
-
CMOS dielectrophoretic Lab-on-Chip platform for manipulation and monitoring of cells.Annu Int Conf IEEE Eng Med Biol Soc. 2015;2015:7530-3. doi: 10.1109/EMBC.2015.7320134. Annu Int Conf IEEE Eng Med Biol Soc. 2015. PMID: 26738034
-
Dual frequency dielectrophoresis with interdigitated sidewall electrodes for microfluidic flow-through separation of beads and cells.Electrophoresis. 2009 Mar;30(5):782-91. doi: 10.1002/elps.200800637. Electrophoresis. 2009. PMID: 19197906
-
Dielectrophoretic platforms for bio-microfluidic systems.Biosens Bioelectron. 2011 Jan 15;26(5):1800-14. doi: 10.1016/j.bios.2010.09.022. Epub 2010 Sep 17. Biosens Bioelectron. 2011. PMID: 20933384 Review.
-
Toward low-voltage dielectrophoresis-based microfluidic systems: A review.Electrophoresis. 2021 Mar;42(5):565-587. doi: 10.1002/elps.202000213. Epub 2020 Nov 22. Electrophoresis. 2021. PMID: 33166414 Review.
Cited by
-
Comparing machine learning and deep learning regression frameworks for accurate prediction of dielectrophoretic force.Sci Rep. 2022 Jul 13;12(1):11971. doi: 10.1038/s41598-022-16114-5. Sci Rep. 2022. PMID: 35831342 Free PMC article.
-
Dielectrophoretic Immobilization of Yeast Cells Using CMOS Integrated Microfluidics.Micromachines (Basel). 2020 May 15;11(5):501. doi: 10.3390/mi11050501. Micromachines (Basel). 2020. PMID: 32429098 Free PMC article.
-
A Prominent Cell Manipulation Technique in BioMEMS: Dielectrophoresis.Micromachines (Basel). 2020 Nov 3;11(11):990. doi: 10.3390/mi11110990. Micromachines (Basel). 2020. PMID: 33153069 Free PMC article. Review.
-
Characterization and Separation of Live and Dead Yeast Cells Using CMOS-Based DEP Microfluidics.Micromachines (Basel). 2021 Mar 6;12(3):270. doi: 10.3390/mi12030270. Micromachines (Basel). 2021. PMID: 33800809 Free PMC article.
-
Dielectrophoretic separation of platelet cells in a microfluidic channel and optimization with fuzzy logic.RSC Adv. 2020 Sep 11;10(56):33731-33738. doi: 10.1039/d0ra06271e. eCollection 2020 Sep 10. RSC Adv. 2020. PMID: 35519028 Free PMC article.
References
-
- Li H., Zheng Y., Akin D., Bashir R. Characterization and modeling of a microfluidic dielectrophoresis filter for biological species. J. Microelectromech. Syst. 2005;15:103–112. doi: 10.1109/JMEMS.2004.839124. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

