Long-Term Clinical Effectiveness of a Drug-Coated Balloon for the Treatment of Femoropopliteal Lesions
- PMID: 31195825
- PMCID: PMC6636795
- DOI: 10.1161/CIRCINTERVENTIONS.118.007702
Long-Term Clinical Effectiveness of a Drug-Coated Balloon for the Treatment of Femoropopliteal Lesions
Abstract
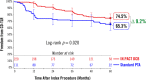
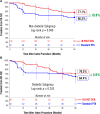
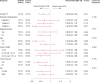
Background While randomized trials have demonstrated the superiority of drug-coated balloon (DCB) angioplasty versus standard percutaneous transluminal angioplasty (PTA) in patients with femoropopliteal peripheral artery disease, the long-term durability of DCB angioplasty remains uncertain. Methods and Results IN.PACT SFA is a prospective, multicenter, randomized single-blinded trial (Randomized Trial of IN.PACT Admiral Paclitaxel-Coated Percutaneous Transluminal Angioplasty [PTA] Balloon Catheter vs Standard PTA for the Treatment of Atherosclerotic Lesions in the Superficial Femoral Artery [SFA] and/or Proximal Popliteal Artery [PPA]) that enrolled 331 subjects with symptomatic (Rutherford 2-4) femoropopliteal lesions. Subjects were randomly assigned 2:1 to the IN.PACT Admiral DCB or PTA. Assessments through 5 years included freedom from clinically driven target lesion revascularization, the primary safety end point, and major adverse events. Through 5 years, patients treated with the IN.PACT Admiral DCB demonstrated a sustained treatment effect with superior freedom from clinically driven target lesion revascularization when compared with PTA (Kaplan-Meier estimate of 74.5% versus 65.3%; log-rank P=0.020). The primary safety composite was achieved in 70.7% of subjects in the DCB and 59.6% in the PTA groups ( P=0.068). The major adverse event rate was 42.9% for DCB and 48.1% for PTA ( P=0.459). There were no device- or procedure-related deaths in either group as adjudicated by an independent and blinded Clinical Events Committee. Conclusions The IN.PACT SFA randomized trial demonstrates that the IN.PACT Admiral DCB continues to perform better than PTA through 5 years with higher freedom from clinically driven target lesion revascularization. The sustained safety and effectiveness profile of this DCB supports its use as a preferred treatment choice compared with PTA for femoropopliteal lesions. Clinical Trial Registration URL: https://www.clinicaltrials.gov . Unique identifier: NCT01175850 (IN.PACT SFA phase I) and NCT01566461 (IN.PACT SFA phase II).
Keywords: angioplasty; drug-coated balloons; paclitaxel; peripheral artery disease.
Figures
Comment in
-
Aere Perennius.Circ Cardiovasc Interv. 2019 Jun;12(6):e008088. doi: 10.1161/CIRCINTERVENTIONS.119.008088. Epub 2019 Jun 14. Circ Cardiovasc Interv. 2019. PMID: 31195823 No abstract available.
References
-
- Laird JR, Schneider PA, Tepe G, Brodmann M, Zeller T, Metzger C, Krishnan P, Scheinert D, Micari A, Cohen DJ, Wang H, Hasenbank MS, Jaff MR IN.PACT SFA Trial Investigators. Durability of treatment effect using a drug-coated balloon for femoropopliteal lesions: 24-month results of IN.PACT SFA. J Am Coll Cardiol. 2015;66:2329–2338. doi: 10.1016/j.jacc.2015.09.063. - PubMed
-
- Tepe G, Laird J, Schneider P, Brodmann M, Krishnan P, Micari A, Metzger C, Scheinert D, Zeller T, Cohen DJ, Snead DB, Alexander B, Landini M, Jaff MR IN.PACT SFA Trial Investigators. Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial. Circulation. 2015;131:495–502. doi: 10.1161/CIRCULATIONAHA.114.011004. - PMC - PubMed
-
- Iida O, Soga Y, Urasawa K, Saito S, Jaff MR, Wang H, Ookubo H, Yokoi H MDT-2113 SFA Japan Investigators. Drug-coated balloon vs standard percutaneous transluminal angioplasty for the treatment of atherosclerotic lesions in the superficial femoral and proximal popliteal arteries: one-year results of the MDT-2113 SFA Japan randomized trial. J Endovasc Ther. 2018;25:109–117. doi: 10.1177/1526602817745565. - PMC - PubMed
-
- Schneider PA, Laird JR, Tepe G, Brodmann M, Zeller T, Scheinert D, Metzger C, Micari A, Sachar R, Jaff MR, Wang H, Hasenbank MS, Krishnan P IN.PACT SFA Trial Investigators. Treatment effect of drug-coated balloons is durable to 3 years in the femoropopliteal arteries: long-term results of the IN.PACT SFA randomized trial. Circ Cardiovasc Interv. 2018;11:e005891. doi: 10.1161/CIRCINTERVENTIONS.117.005891. - PMC - PubMed
-
- Krishnan P, Faries P, Niazi K, Jain A, Sachar R, Bachinsky WB, Cardenas J, Werner M, Brodmann M, Mustapha JA, Mena-Hurtado C, Jaff MR, Holden AH, Lyden SP. Stellarex drug-coated balloon for treatment of femoropopliteal disease: twelve-month outcomes from the randomized ILLUMENATE pivotal and pharmacokinetic studies. Circulation. 2017;136:1102–1113. doi: 10.1161/CIRCULATIONAHA.117.028893. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

