Repositioning rifamycins for Mycobacterium abscessus lung disease
- PMID: 31195849
- PMCID: PMC6663560
- DOI: 10.1080/17460441.2019.1629414
Repositioning rifamycins for Mycobacterium abscessus lung disease
Abstract
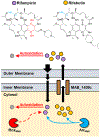
Introduction: The treatment of Mycobacterium abscessus lung disease faces significant challenges due to intrinsic antibiotic resistance. New drugs are needed to cure this incurable disease. The key anti-tubercular rifamycin, rifampicin, suffers from low potency against M. abscessus and is not used clinically. Recently, another member of the rifamycin class, rifabutin, was shown to be active against the opportunistic pathogen. Areas covered: In this review, the authors discuss the rifamycins as a reemerging drug class for treating M. abscessus infections. The authors focus on the differential potency of rifampicin and rifabutin against M. abscessus in the context of intrinsic antibiotic resistance and bacterial uptake and metabolism. Reports of rifamycin-based drug synergies and rifamycin potentiation by host-directed therapy are evaluated. Expert opinion: While repurposing rifabutin for M. abscessus lung disease may provide some immediate relief, the repositioning (chemical optimization) of rifamycins offers long-term potential for improving clinical outcomes. Repositioning will require a multifaceted approach involving renewed screening of rifamycin libraries, medicinal chemistry to improve 'bacterial cell pharmacokinetics', better models of bacterial pathophysiology and infection, and harnessing of drug synergies and host-directed therapy towards the development of a better drug regimen.
Keywords: Lung disease; Mycobacterium abscessus; non-tuberculous mycobacteria; repositioning; rifamycins.
Conflict of interest statement
Declaration of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures
Similar articles
-
Rifabutin Is Active against Mycobacterium abscessus in Mice.Antimicrob Agents Chemother. 2020 Jan 27;64(2):e01943-19. doi: 10.1128/AAC.01943-19. Print 2020 Jan 27. Antimicrob Agents Chemother. 2020. PMID: 31767722 Free PMC article.
-
Blocking Bacterial Naphthohydroquinone Oxidation and ADP-Ribosylation Improves Activity of Rifamycins against Mycobacterium abscessus.Antimicrob Agents Chemother. 2021 Aug 17;65(9):e0097821. doi: 10.1128/AAC.00978-21. Epub 2021 Aug 17. Antimicrob Agents Chemother. 2021. PMID: 34228543 Free PMC article.
-
Next-generation rifamycins for the treatment of mycobacterial infections.Proc Natl Acad Sci U S A. 2025 May 6;122(18):e2423842122. doi: 10.1073/pnas.2423842122. Epub 2025 May 1. Proc Natl Acad Sci U S A. 2025. PMID: 40310456 Free PMC article.
-
Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus.Nat Rev Microbiol. 2020 Jul;18(7):392-407. doi: 10.1038/s41579-020-0331-1. Epub 2020 Feb 21. Nat Rev Microbiol. 2020. PMID: 32086501 Review.
-
The treatment of rapidly growing mycobacterial infections.Clin Chest Med. 2015 Mar;36(1):67-78. doi: 10.1016/j.ccm.2014.10.004. Epub 2014 Nov 5. Clin Chest Med. 2015. PMID: 25676520 Review.
Cited by
-
Opportunist Coinfections by Nontuberculous Mycobacteria and Fungi in Immunocompromised Patients.Antibiotics (Basel). 2020 Nov 2;9(11):771. doi: 10.3390/antibiotics9110771. Antibiotics (Basel). 2020. PMID: 33147819 Free PMC article. Review.
-
Artemisia annua and Artemisia afra extracts exhibit strong bactericidal activity against Mycobacterium tuberculosis.J Ethnopharmacol. 2020 Nov 15;262:113191. doi: 10.1016/j.jep.2020.113191. Epub 2020 Jul 27. J Ethnopharmacol. 2020. PMID: 32730878 Free PMC article.
-
C25-modified rifamycin derivatives with improved activity against Mycobacterium abscessus.PNAS Nexus. 2022 Aug 9;1(4):pgac130. doi: 10.1093/pnasnexus/pgac130. eCollection 2022 Sep. PNAS Nexus. 2022. PMID: 36714853 Free PMC article.
-
Exploring antibiotic resistance mechanisms in Mycobacterium abscessus for enhanced therapeutic approaches.Front Microbiol. 2024 Feb 6;15:1331508. doi: 10.3389/fmicb.2024.1331508. eCollection 2024. Front Microbiol. 2024. PMID: 38380095 Free PMC article. Review.
-
Rifabutin Is Active against Mycobacterium abscessus in Mice.Antimicrob Agents Chemother. 2020 Jan 27;64(2):e01943-19. doi: 10.1128/AAC.01943-19. Print 2020 Jan 27. Antimicrob Agents Chemother. 2020. PMID: 31767722 Free PMC article.
References
-
- Tortoli E Chapter 1 - The Taxonomy of the Genus Mycobacterium In: Velayati AA, Farnia P, editors. Nontuberculous Mycobacteria (NTM): Academic Press; 2019. p. 1–10.
-
- Daniel-Wayman S, Adjemian J, Rebecca Prevots D. Epidemiology of Nontuberculous Mycobacterial Pulmonary Disease (NTM PD) in the USA In: Griffith DE, editor. Nontuberculous Mycobacterial Disease: A Comprehensive Approach to Diagnosis and Management. Cham: Springer International Publishing; 2019. p. 145–161.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
