A phase II randomized placebo-controlled double-blind study of salvage radiation therapy plus placebo versus SRT plus enzalutamide with high-risk PSA-recurrent prostate cancer after radical prostatectomy (SALV-ENZA)
- PMID: 31196032
- PMCID: PMC6567492
- DOI: 10.1186/s12885-019-5805-z
A phase II randomized placebo-controlled double-blind study of salvage radiation therapy plus placebo versus SRT plus enzalutamide with high-risk PSA-recurrent prostate cancer after radical prostatectomy (SALV-ENZA)
Abstract
Background: In men with a rising PSA following radical prostatectomy, salvage radiation therapy (SRT) offers a second chance for cure. Hormonal therapy can be combined with SRT in order to increase prostate tumor control, albeit with associated higher rates of treatment side effects. This trial studies the effectiveness of SRT combined with hormonal therapy using a more potent anti-androgen with a favorable side effect profile. Enzalutamide, a next generation selective androgen receptor antagonist, is approved by the Food and Drug Administration for the treatment of metastatic castrate-resistant prostate cancer (CRPC) where it has been shown to improve overall survival in combination with androgen deprivation therapy. The primary objective of this study is to evaluate the efficacy of combination SRT and enzalutamide for freedom-from-PSA-progression. Secondary objectives include time to local recurrence within the radiation field, metastasis-free survival and safety as determined by frequency and severity of adverse events.
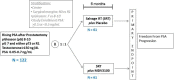
Methods/design: This is a randomized, double-blind, phase II, prospective, multicenter study in adult males with biochemically recurrent prostate cancer following radical prostatectomy. Following registration, enzalutamide 160 mg or placebo by mouth (PO) once daily will be administered for 6 months. Following two months of study drug, external beam radiotherapy to 66.6-70.2 Gray (Gy) will be administered to the prostate bed over 7-8 weeks while continuing daily placebo/enzalutamide. This is followed by two additional months of placebo/enzalutamide.
Discussion: The SALV-ENZA trial is the first phase II placebo-controlled double-blinded randomized study to test SRT in combination with a next generation androgen receptor antagonist in men with high-risk recurrent prostate cancer after radical prostatectomy. The primary hypothesis of this study is that clinical outcomes will be improved by the addition of enzalutamide compared to standard-of-care SRT alone and pave the path for phase III evaluation of this combination.
Trial registrations: ClinicaltTrials.gov Identifier: NCT02203695 Date of Registration: 06/16/2014. Date of First Participant Enrollment: 04/16/2015.
Keywords: Enzalutamide; High-risk prostate cancer; Prostatectomy; Recurrent prostate cancer; Salvage radiation therapy (SRT).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Phase II Randomized Study of Salvage Radiation Therapy Plus Enzalutamide or Placebo for High-Risk Prostate-Specific Antigen Recurrent Prostate Cancer After Radical Prostatectomy: The SALV-ENZA Trial.J Clin Oncol. 2023 Feb 20;41(6):1307-1317. doi: 10.1200/JCO.22.01662. Epub 2022 Nov 11. J Clin Oncol. 2023. PMID: 36367998 Free PMC article. Clinical Trial.
-
Abiraterone acetate and prednisolone with or without enzalutamide for high-risk non-metastatic prostate cancer: a meta-analysis of primary results from two randomised controlled phase 3 trials of the STAMPEDE platform protocol.Lancet. 2022 Jan 29;399(10323):447-460. doi: 10.1016/S0140-6736(21)02437-5. Epub 2021 Dec 23. Lancet. 2022. PMID: 34953525 Free PMC article.
-
Enzalutamide in Men with Nonmetastatic, Castration-Resistant Prostate Cancer.N Engl J Med. 2018 Jun 28;378(26):2465-2474. doi: 10.1056/NEJMoa1800536. N Engl J Med. 2018. PMID: 29949494 Free PMC article. Clinical Trial.
-
Enzalutamide for the treatment of nonmetastatic castration-resistant prostate cancer.Expert Opin Pharmacother. 2020 Dec;21(17):2091-2099. doi: 10.1080/14656566.2020.1803281. Epub 2020 Aug 12. Expert Opin Pharmacother. 2020. PMID: 32783772 Review.
-
Safety and effectiveness of enzalutamide in men with metastatic, castration-resistant prostate cancer.Expert Opin Pharmacother. 2015 Apr;16(5):749-54. doi: 10.1517/14656566.2015.1016911. Epub 2015 Feb 17. Expert Opin Pharmacother. 2015. PMID: 25687355 Review.
Cited by
-
Should androgen deprivation therapy and other systemic treatments be used in men with prostate cancer and a rising PSA post-local treatments?Ther Adv Med Oncol. 2021 Oct 20;13:17588359211051870. doi: 10.1177/17588359211051870. eCollection 2021. Ther Adv Med Oncol. 2021. PMID: 34707693 Free PMC article. Review.
References
-
- Spratt DE, Dess RT, Zumsteg ZS, Lin DW, Tran PT, Morgan TM, Antonarakis ES, Nguyen PL, Ryan CJ, Sandler HM, et al. A systematic review and framework for the use of hormone therapy with salvage radiation therapy for recurrent prostate Cancer. Eur Urol. 2018;73(2):156–165. doi: 10.1016/j.eururo.2017.06.027. - DOI - PubMed
-
- Anscher MS, Clough R, Robertson CN, Prosnitz LR, Dahm P, Walther P, Donatucci CF, Albala DM, Febbo P, George DJ, et al. Timing and patterns of recurrences and deaths from prostate cancer following adjuvant pelvic radiotherapy for pathologic stage T3/4 adenocarcinoma of the prostate. Prostate Cancer Prostatic Dis. 2006;9(3):254–260. doi: 10.1038/sj.pcan.4500903. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous