Optimal indication criteria for neoadjuvant chemotherapy in patients with resectable colorectal liver metastases
- PMID: 31196104
- PMCID: PMC6567619
- DOI: 10.1186/s12957-019-1641-5
Optimal indication criteria for neoadjuvant chemotherapy in patients with resectable colorectal liver metastases
Abstract
Background: There are no optimal indication criteria for neoadjuvant chemotherapy (NAC) in patients with resectable colorectal liver metastases (CLM). The aim of this study was to prospectively assess the survival benefit of selective NAC administration in this patient population based on tumor characteristics.
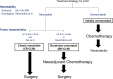
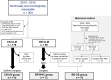
Methods: Borderline resectable CLM (BR-CLM) were defined as four or more liver metastases, CLM larger than 5 cm, or CLM with concomitant resectable extrahepatic metastases. From 2010 to 2015, NAC was administered to BR-CLM patients. Upfront surgery without NAC was performed to patients having clearly resectable CLM (less than 3 lesions, smaller than 5 cm, and no extrahepatic metastases: CR-US group). Survival outcomes of the two groups were assessed.
Results: The BR-NAC group comprised 73 patients and the CR-US group 172. All patients in the BR-NAC group underwent subsequent resection, as none showed disease progression or chemotherapy-associated liver damage. The 3- and 5-year overall survival rates of the CR-US group were 83.0% and 74.0%, while patients in the BR-NAC group had comparable 3-year and 5-year overall survivals (80.5% and 66.6%, P = 0.397).
Conclusion: Defining BR-CLM based on tumor characteristics optimizes patient selection for NAC. Favorable overall survival can be achieved by upfront surgery in patients with clearly resectable CLM and by NAC in patients with BR-CLM.
Keywords: Chemotherapy; Colorectal cancer; Liver metastases; Liver resection.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Adam René, Wicherts Dennis A., de Haas Robbert J., Ciacio Oriana, Lévi Francis, Paule Bernard, Ducreux Michel, Azoulay Daniel, Bismuth Henri, Castaing Denis. Patients With Initially Unresectable Colorectal Liver Metastases: Is There a Possibility of Cure? Journal of Clinical Oncology. 2009;27(11):1829–1835. doi: 10.1200/JCO.2008.19.9273. - DOI - PubMed
-
- Folprecht G, Gruenberger T, Bechstein W, Raab HR, Weitz J, Lordick F, et al. Survival of patients with initially unresectable colorectal liver metastases treated with FOLFOX/cetuximab or FOLFIRI/cetuximab in a multidisciplinary concept (CELIM study) Ann Oncol. 2014;25(5):1018–1025. doi: 10.1093/annonc/mdu088. - DOI - PubMed
-
- Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371(9617):1007–1016. doi: 10.1016/S0140-6736(08)60455-9. - DOI - PMC - PubMed
-
- Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14(12):1208–1215. doi: 10.1016/S1470-2045(13)70447-9. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

