The impact of sirolimus therapy on lesion size, clinical symptoms, and quality of life of patients with lymphatic anomalies
- PMID: 31196128
- PMCID: PMC6567608
- DOI: 10.1186/s13023-019-1118-1
The impact of sirolimus therapy on lesion size, clinical symptoms, and quality of life of patients with lymphatic anomalies
Abstract
Background: Lymphatic anomalies (LAs) include several disorders in which abnormal lymphatic tissue invades the neck, chest, and various organs. Progressive cases may result in lethal outcomes and have proven difficult to treat. Sirolimus is showing promising results in the management of vascular anomalies. We examined the efficacy and safety of sirolimus treatment in patients with progressive LAs.
Methods: All patients with LAs treated with sirolimus from May 2015 to September 2018 were included. They received oral sirolimus once a day and the dose was adjusted so that the trough concentration remained within 5-15 ng/mL. We prospectively reviewed the response to drugs (the response rate of radiological volumetric change of the target lesion), severity scores, reported quality of life (QOL), and adverse effects at 6 months after administration.
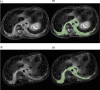
Results: Twenty patients (five with cystic lymphatic malformation (LM), three with kaposiform lymphangiomatosis, three with generalized lymphatic anomaly, six with Gorham-Stout disease, and three with central conducting lymphatic anomaly) were treated with sirolimus at our institution. Fifty percent of patients (10/20) demonstrated a partial response by a radiological examination and a significant improvement in disease severity and QOL scores (P = 0.0020 and P = 0.0117, respectively). Ten patients who had no reduction in lesion size (stable disease group) showed no significant improvement in disease severity and QOL scores. Eighty percent of patients (16/20) had side effects, such as stomatitis, infection, and hyperlipidemia.
Conclusions: Sirolimus impacts the reduction of the lymphatic tissue volume of LMs and could lead to improvement in clinical symptoms and QOL.
Trial registration: UMIN Clinical Trials Registry, UMIN000016580 . Registered 19 February 2015.
Keywords: Generalized lymphatic anomaly; Gorham-stout disease; Lymphatic malformation; Mammalian target of rapamycin; Vascular malformations.
Conflict of interest statement
M.O. and T.F. received research funding from Nobelpharma. Sirolimus tablets were supplied by Nobelpharma. The other authors declare no competing interests.
Figures


Similar articles
-
Efficacy of systemic sirolimus in the treatment of generalized lymphatic anomaly and Gorham-Stout disease.Pediatr Blood Cancer. 2019 May;66(5):e27614. doi: 10.1002/pbc.27614. Epub 2019 Jan 22. Pediatr Blood Cancer. 2019. PMID: 30672136 Free PMC article. Clinical Trial.
-
Sirolimus in the Treatment of Vascular Anomalies.Eur J Pediatr Surg. 2017 Feb;27(1):86-90. doi: 10.1055/s-0036-1593383. Epub 2016 Oct 10. Eur J Pediatr Surg. 2017. PMID: 27723921
-
Sirolimus treatment for intractable lymphatic anomalies: an open-label, single-arm, multicenter, prospective trial.Front Med (Lausanne). 2024 Feb 8;11:1335469. doi: 10.3389/fmed.2024.1335469. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38390569 Free PMC article.
-
Treatment of Lymphatic Malformations with the mTOR Inhibitor Sirolimus: A Systematic Review.Lymphat Res Biol. 2018 Aug;16(4):330-339. doi: 10.1089/lrb.2017.0062. Epub 2018 Jun 20. Lymphat Res Biol. 2018. PMID: 29924669
-
A narrative review of the role of sirolimus in the treatment of congenital vascular malformations.J Vasc Surg Venous Lymphat Disord. 2021 Sep;9(5):1321-1333. doi: 10.1016/j.jvsv.2021.03.001. Epub 2021 Mar 15. J Vasc Surg Venous Lymphat Disord. 2021. PMID: 33737259 Review.
Cited by
-
Evaluation of Clinical Findings in Children with Chylothorax: A Descriptive Study.Turk Arch Pediatr. 2023 Jan;58(1):28-33. doi: 10.5152/TurkArchPediatr.2022.22182. Turk Arch Pediatr. 2023. PMID: 36598208 Free PMC article.
-
Clinical approach for pulmonary lymphatic disorders.World J Clin Cases. 2024 Sep 26;12(27):6020-6026. doi: 10.12998/wjcc.v12.i27.6020. World J Clin Cases. 2024. PMID: 39328863 Free PMC article.
-
Sirolimus treatment for paediatric head and neck lymphatic malformations: a systematic review.Eur Arch Otorhinolaryngol. 2023 Aug;280(8):3529-3540. doi: 10.1007/s00405-023-07991-1. Epub 2023 Apr 28. Eur Arch Otorhinolaryngol. 2023. PMID: 37115326 Free PMC article.
-
Population pharmacokinetic study in children with vascular anomalies: body weight as a key variable in predicting the initial dose and dosing frequency of sirolimus.Front Pharmacol. 2024 Sep 24;15:1457614. doi: 10.3389/fphar.2024.1457614. eCollection 2024. Front Pharmacol. 2024. PMID: 39380905 Free PMC article.
-
Gorham-Stout Disease with Multiple Bone Involvement-Challenging Diagnosis of a Rare Disease and Literature Review.Medicina (Kaunas). 2021 Jul 2;57(7):681. doi: 10.3390/medicina57070681. Medicina (Kaunas). 2021. PMID: 34356962 Free PMC article. Review.
References
-
- International Society for the Study of Vascular Anomalies: ISSVA classification for Vascular Anomalies (approved at the May 2018 General Assembly in Amsterdam, the Netherlands) http://issva.org/classification (Accessed June 2018).
-
- Foley LS, Kulungowski AM. Vascular anomalies in pediatrics. Adv Pediatr Infect Dis. 2015;62:227–255. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

