Advancing Symptom Alleviation with Palliative Treatment (ADAPT) trial to improve quality of life: a study protocol for a randomized clinical trial
- PMID: 31196156
- PMCID: PMC6567600
- DOI: 10.1186/s13063-019-3417-1
Advancing Symptom Alleviation with Palliative Treatment (ADAPT) trial to improve quality of life: a study protocol for a randomized clinical trial
Abstract
Background: People living with chronic heart failure (CHF), chronic obstructive pulmonary disease (COPD), and interstitial lung disease (ILD) suffer impaired quality of life due to burdensome symptoms and depression. The Advancing Symptom Alleviation with Palliative Treatment (ADAPT) trial aims to determine the effect of a multidisciplinary, team-based intervention on quality of life in people with these common diseases.
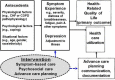
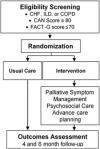
Methods/design: The ADAPT trial is a two-site, patient-level randomized clinical trial that examines the effectiveness of the ADAPT intervention compared to usual care on patient-reported quality of life at 6 months in veterans with CHF, COPD or ILD with poor quality of life and increased risk for hospitalization or death. The ADAPT intervention involves a multidisciplinary team-a registered nurse, social worker, palliative care specialist, and primary care provider (with access to a pulmonologist and cardiologist)-who meet weekly to make recommendations and write orders for consideration by participants' individual primary care providers. The nurse and social worker interact with participants over six visits to identify and manage a primary bothersome symptom and complete a structured psychosocial intervention and advance care planning. The primary outcome is change in patient-reported quality of life at 6 months as measured by the Functional Assessment of Chronic Illness Therapy-General questionnaire. Secondary outcomes at 6 months include change in symptom distress, depression, anxiety, disease-specific quality of life hospitalizations, and advance care planning communication and documentation. Intervention implementation will be assessed using a mixed-methods approach including a qualitative assessment of participants' and intervention personnel experiences and a quantitative assessment of care delivery, resources, and cost.
Discussion: The ADAPT trial studies an innovative intervention designed to improve quality of life for veterans with common, burdensome illnesses by targeting key underlying factors-symptoms and depression-that impair quality of life but persist despite disease-specific therapies. Leveraging the skills of affiliate health providers with physician supervision will extend the reach of palliative care and improve quality of life for those with advanced disease within routine outpatient care. The hybrid effectiveness/implementation design of the ADAPT trial will shorten the time to broader dissemination if effective and create avenues for future research.
Trial registration: ClinicalTrials.gov, NCT02713347 . Registered March 19, 2016.
Keywords: Heart failure; chronic obstructive pulmonary disease; interstitial lung disease; palliative care; quality of life.
Conflict of interest statement
The authors declare they have no competing interests.
Figures
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical