Safety and efficacy of ex vivo expanded CD34+ stem cells in murine and primate models
- PMID: 31196160
- PMCID: PMC6567473
- DOI: 10.1186/s13287-019-1275-0
Safety and efficacy of ex vivo expanded CD34+ stem cells in murine and primate models
Abstract
Background: Hematopoietic stem cell (HSC) transplantation has been widely applied to the treatment of malignant blood diseases. However, limited number of functional HSCs hinders successful transplantation. The purpose of our current study is to develop a new and cost-efficient medium formulation that could greatly enhance the expansion of HSCs while retaining their long-term repopulation and hematopoietic properties for effective clinical transplantation.
Methods: Enriched human CD34+ cells and mobilized nonhuman primate peripheral blood CD34+ cells were expanded with a new, cost-efficient expansion medium formulation, named hematopoietic expansion medium (HEM), consisting of various cytokines and nutritional supplements. The long-term repopulation potential and hematologic-lineage differentiation ability of expanded human cells were studied in the non-obese diabetic/severe combined immunodeficiency mouse model. Furthermore, the efficacy and safety studies were performed by autologous transplantation of expanded primate cells in the nonhuman primate model.
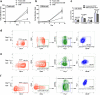
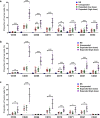
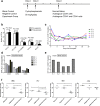
Results: HEM could effectively expand human CD34+ cells by up to 129 fold within 9 days. Expanded HSCs retained long-term repopulation potential and hematologic-lineage differentiation ability, as indicated by (1) maintenance (over unexpanded HSCs) of immunophenotypes of CD38-CD90+CD45RA-CD49f+ in CD34+ cells after expansion; (2) significant presence of multiple human hematopoietic lineages in mouse peripheral blood and bone marrow following primary transplantation; (3) enrichment (over unexpanded HSCs) in SCID-repopulating cell frequency measured by limiting dilution analysis; and (4) preservation of both myeloid and lymphoid potential among human leukocytes from mouse bone marrow in week 24 after primary transplantation or secondary transplantation. Moreover, the results of autologous transplantation in nonhuman primates demonstrated that HEM-expanded CD34+ cells could enhance hematological recovery after myelo-suppression. All primates transplanted with the expanded autologous CD34+ cells survived for over 18 months without any noticeable abnormalities.
Conclusions: Together, these findings demonstrate promising potential for the utility of HEM to improve expansion of HSCs for clinical application.
Keywords: Ex vivo expansion; Human cord blood; Long-term HSC; NOD/SCID mice; Nonhuman primates; Transplantation.
Conflict of interest statement
XG and ZR are employees of Biopharmagen Corp. The other authors declare that they have no competing interests.
Figures


Similar articles
-
Ex Vivo Expansion of CD34+ CD90+ CD49f+ Hematopoietic Stem and Progenitor Cells from Non-Enriched Umbilical Cord Blood with Azole Compounds.Stem Cells Transl Med. 2018 May;7(5):376-393. doi: 10.1002/sctm.17-0251. Epub 2018 Feb 2. Stem Cells Transl Med. 2018. PMID: 29392885 Free PMC article.
-
Ex-vivo expansion of nonhuman primate CD34+ cells by stem cell factor Sall4B.Stem Cell Res Ther. 2016 Oct 20;7(1):152. doi: 10.1186/s13287-016-0413-1. Stem Cell Res Ther. 2016. PMID: 27765075 Free PMC article.
-
Different Human Immune Lineage Compositions Are Generated in Non-Conditioned NBSGW Mice Depending on HSPC Source.Front Immunol. 2020 Oct 19;11:573406. doi: 10.3389/fimmu.2020.573406. eCollection 2020. Front Immunol. 2020. PMID: 33193358 Free PMC article.
-
Human CD34-negative hematopoietic stem cells: The current understanding of their biological nature.Exp Hematol. 2021 Apr;96:13-26. doi: 10.1016/j.exphem.2021.02.004. Epub 2021 Feb 19. Exp Hematol. 2021. PMID: 33610645 Review.
-
A sticky wicket: Defining molecular functions for CD34 in hematopoietic cells.Exp Hematol. 2020 Jun;86:1-14. doi: 10.1016/j.exphem.2020.05.004. Epub 2020 May 16. Exp Hematol. 2020. PMID: 32422232 Review.
Cited by
-
Co-culture of mesenchymal stem cell spheres with hematopoietic stem cells under hypoxia: a cost-effective method to maintain self-renewal and homing marker expression.Mol Biol Rep. 2022 Feb;49(2):931-941. doi: 10.1007/s11033-021-06912-x. Epub 2021 Nov 6. Mol Biol Rep. 2022. PMID: 34741711
-
Overcoming the UCB HSCs -Derived NK cells Dysfunction through Harnessing RAS/MAPK, IGF-1R and TGF-β Signaling Pathways.Cancer Cell Int. 2021 Jun 7;21(1):298. doi: 10.1186/s12935-021-01983-z. Cancer Cell Int. 2021. PMID: 34098947 Free PMC article.
-
Delayed effects of radiation in adipose tissue reflect progenitor damage and not cellular senescence.Geroscience. 2023 Feb;45(1):507-521. doi: 10.1007/s11357-022-00660-x. Epub 2022 Sep 22. Geroscience. 2023. PMID: 36136223 Free PMC article.
-
Long-Term Human Hematopoietic Stem Cell Culture in Microdroplets.Micromachines (Basel). 2021 Jan 16;12(1):90. doi: 10.3390/mi12010090. Micromachines (Basel). 2021. PMID: 33467039 Free PMC article.
-
CH02 peptide promotes ex vivo expansion of umbilical cord blood-derived CD34 + hematopoietic stem/progenitor cells.Acta Biochim Biophys Sin (Shanghai). 2023 Oct 25;55(10):1630-1639. doi: 10.3724/abbs.2023047. Acta Biochim Biophys Sin (Shanghai). 2023. PMID: 37381672 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

