Examining the fundamental biology of a novel population of directly reprogrammed human neural precursor cells
- PMID: 31196173
- PMCID: PMC6567617
- DOI: 10.1186/s13287-019-1255-4
Examining the fundamental biology of a novel population of directly reprogrammed human neural precursor cells
Abstract
Background: Cell reprogramming is a promising avenue for cell-based therapies as it allows for the generation of multipotent, unipotent, or mature somatic cells without going through a pluripotent state. While the use of autologous cells is considered ideal, key challenges for their clinical translation include the ability to reproducibly generate sufficient quantities of cells within a therapeutically relevant time window.
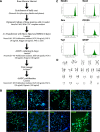
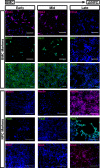
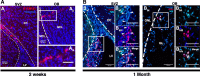
Methods: We performed transfection of three distinct human somatic starting populations of cells with a non-integrating synthetic plasmid expressing Musashi 1 (MSI1), Neurogenin 2 (NGN2), and Methyl-CpG-Binding Domain 2 (MBD2). The resulting directly reprogrammed neural precursor cells (drNPCs) were examined in vitro using RT-qPCR, karyotype analysis, immunohistochemistry, and FACS at early and late time post-transfection. Electrophysiology (patch clamp) was performed on drNPC-derived neurons to determine their capacity to generate action potentials. In vivo characterization was performed following transplantation of drNPCs into two animal models (Shiverer and SCID/Beige mice), and the numbers, location, and differentiation profile of the transplanted cells were examined using immunohistochemistry.
Results: Human somatic cells can be directly reprogrammed within two weeks to neural precursor cells (drNPCs) by transient exposure to Msi1, Ngn2, and MBD2 using non-viral constructs. The drNPCs generate all three neural cell types (astrocytes, oligodendrocytes, and neurons) and can be passaged in vitro to generate large numbers of cells within four weeks. drNPCs can respond to in vivo differentiation and migration cues as demonstrated by their migration to the olfactory bulb and contribution to neurogenesis in vivo. Differentiation profiles of transplanted cells onto the corpus callosum of myelin-deficient mice reveal the production of oligodendrocytes and astrocytes.
Conclusions: Human drNPCs can be efficiently and rapidly produced from donor somatic cells and possess all the important characteristics of native neural multipotent cells including differentiation into neurons, astrocytes, and oligodendrocytes, and in vivo neurogenesis and myelination.
Keywords: Direct reprogramming; In vivo neurogenesis; In vivo remyelination; Neural precursor cells; Neural stem cells; drNPC.
Conflict of interest statement
JEA is a shareholder of New World Laboratories. RE, CB, SM, and OM are employees of New World Laboratories. The other authors declare that they have no competing interests.
Figures



References
-
- Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Morshead CM, Fehlings MG. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci. 2006;26:3377–3389. doi: 10.1523/JNEUROSCI.4184-05.2006. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

