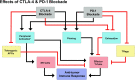
Combination of CTLA-4 and PD-1 blockers for treatment of cancer
- PMID: 31196207
- PMCID: PMC6567914
- DOI: 10.1186/s13046-019-1259-z
Combination of CTLA-4 and PD-1 blockers for treatment of cancer
Abstract
Targeting checkpoints of immune cell activation has been demonstrated to be the most effective approach for activation of anti-tumor immune responses. Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1), both inhibitory checkpoints commonly seen on activated T-cells have been found to be the most reliable targets for the treatment of cancer. Six drugs targeting PD-1 or its ligand PD-L1 and one drug targeting CTLA-4 have been approved for treatment of different types of cancers and several others are in advanced stages of development. The drugs when administered as monotherapy had dramatic increase in durable response rates and had manageable safety profile, but more than 50% of patients failed to respond to treatment. Combination of CTLA-4 and PD-1 blockers was then evaluated to increase the response rates in patients, and ipilimumab (anti-CTLA-4) plus nivolumab (anti-PD-1) combination was shown to significantly enhance efficacy in metastatic melanoma patients. Subsequently, ipilimumab plus nivolumab was approved for treatment of metastatic melanoma, advanced renal cell carcinoma and metastatic colorectal cancer with MMR/MSI-H aberrations. The success of combination encouraged multiple clinical studies in other cancer types. Efficacy of the combination has been shown in a number of published studies and is under evaluation in multiple ongoing studies. This review aims to support future research in combination immunotherapy by discussing the basic details of CTLA-4 and PD-1 pathways and the results from clinical studies that evaluated combination of CTLA-4 and PD-1/PD-L1 blockers.
Keywords: CTLA-4; Combination therapy; Immunotherapy; PD-1.
Conflict of interest statement
The author declares that he/she has no competing interests.
Figures
References
-
- Rotte A, Bhandaru M. Interleukin-2. Immunotherapy of melanoma. Cham: Springer International Publishing; 2016.
-
- Rotte A, Bhandaru M. Interferon-a2b. Immunotherapy of melanoma. Cham: Springer International Publishing; 2016.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials