Curcumin overcome primary gefitinib resistance in non-small-cell lung cancer cells through inducing autophagy-related cell death
- PMID: 31196210
- PMCID: PMC6567416
- DOI: 10.1186/s13046-019-1234-8
Curcumin overcome primary gefitinib resistance in non-small-cell lung cancer cells through inducing autophagy-related cell death
Abstract
Background: Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) are being wildly used as target therapy in non-small-cell lung cancer (NSCLC). However, NSCLC patients with wild-type EGFR and KRAS mutation are primary resistant to EGFR-TKIs such as gefitinib. Curcumin has been known as a potential therapeutic agent for several major human cancers. In this study, we investigated the effect of curcumin on the reversal of gefitinib resistance in NSCLC cells as well as their molecular bases.
Methods: H157 (wild-type EGFR and KARS mutation) and H1299 (wild-type EGFR and HRAS mutation) cells were treated with gefitinib or curcumin alone, or the two combination, and then cell viability, EGFR activity, expressions of Sp1 and Sp1-dependent proteins and receptor tyrosine kinases, markers of autophagy and apoptosis were examined by using CCK-8, colony formation, immunoblot, quantitative PCR, immunofluoscence, and flow cytometry assays. Also xenograft experiments were conduced to test the synergism of curcumin to gefitinib.
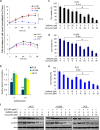
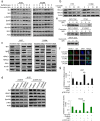
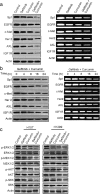
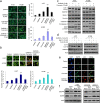
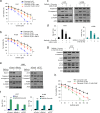
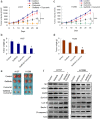
Results: Our results showed that curcumin significantly enhanced inhibitory effect of gefitinib on primary gefitinib-resistant NSCLC cell lines H157 and H1299. Combination treatment with curcumin and gefitinib markedly downregulated EGFR activity through suppressing Sp1 and blocking interaction of Sp1 and HADC1, and markedly suppressed receptor tyrosine kinases as well as ERK/MEK and AKT/S6K pathways in the resistant NSCLC cells. Meanwhile, combination treatment of curcumin and gefitinib caused dramatic autophagy induction, autophagic cell death and autophagy-mediated apoptosis, compared to curcumin or gefitinib treatment alone, as evidenced by the findings that curcumin and gefitinib combination treatment-produced synergistic growth inhibition and apoptosis activation can be reversed by pharmacological autophagy inhibitors (Baf A1 or 3-MA) or knockdown of Beclin-1 or ATG7, also can be partially returned by pan-caspase inhibitor (Z-VAD-FMK) in H157 and H1299 cells. Xenograft experiments in vivo yielded similar results.
Conclusions: These data indicate that the synergism of curcumin on gefitinib was autophagy dependent. Curcumin can be used as a sensitizer to enhance the efficacy of EGFR-TKIs and overcome the EGFR-TKI resistance in NSCLC patients with wild-type EGFR and/or KRAS mutation.
Keywords: Curcumin; Epidermal growth factor receptor (EGFR); Gefitinib; Non-small-cell lung cancer; Resistance; Tyrosine kinase inhibitor (TKI).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

