Ion channels and transporters in diabetic kidney disease
- PMID: 31196609
- PMCID: PMC6815098
- DOI: 10.1016/bs.ctm.2019.01.001
Ion channels and transporters in diabetic kidney disease
Abstract
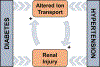
Type 1 and 2 diabetes mellitus are major medical epidemics affecting millions of patients worldwide. Diabetes mellitus is the leading cause of diabetic kidney disease (DKD), which is the most common cause of end-stage renal disease (ESRD). DKD is associated with significant changes in renal hemodynamics and electrolyte transport. Alterations in renal ion transport triggered by pathophysiological conditions in diabetes can exacerbate hypertension, accelerate renal injury, and are integral to the development of DKD. Renal ion transporters and electrolyte homeostasis play a fundamental role in functional changes and injury to the kidney during DKD. With the large number of ion transporters involved in DKD, understanding the roles of individual transporters as well as the complex cascades through which they interact is essential in the development of effective treatments for patients suffering from this disease. This chapter aims to gather current knowledge of the major renal ion transporters with altered expression and activity under diabetic conditions, and provide a comprehensive overview of their interactions and collective functions in DKD.
Keywords: Diabetic kidney disease; Diabetic nephropathy; ENaC; K(ATP) channel; NHE; SGLT2; TRPC6; TRPM6.
© 2019 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Development and internal validation of machine learning algorithms for end-stage renal disease risk prediction model of people with type 2 diabetes mellitus and diabetic kidney disease.Ren Fail. 2022 Dec;44(1):562-570. doi: 10.1080/0886022X.2022.2056053. Ren Fail. 2022. PMID: 35373711 Free PMC article.
-
Effects of metabolic memory on inflammation and fibrosis associated with diabetic kidney disease: an epigenetic perspective.Clin Epigenetics. 2021 Apr 21;13(1):87. doi: 10.1186/s13148-021-01079-5. Clin Epigenetics. 2021. PMID: 33883002 Free PMC article. Review.
-
Role of TRPC6 in Progression of Diabetic Kidney Disease.Curr Hypertens Rep. 2019 May 21;21(7):48. doi: 10.1007/s11906-019-0960-9. Curr Hypertens Rep. 2019. PMID: 31115705 Free PMC article. Review.
-
Ion homeostasis in diabetic kidney disease.Trends Endocrinol Metab. 2024 Feb;35(2):142-150. doi: 10.1016/j.tem.2023.09.009. Epub 2023 Oct 23. Trends Endocrinol Metab. 2024. PMID: 37880052 Review.
-
What's New in the Molecular Mechanisms of Diabetic Kidney Disease: Recent Advances.Int J Mol Sci. 2022 Dec 29;24(1):570. doi: 10.3390/ijms24010570. Int J Mol Sci. 2022. PMID: 36614011 Free PMC article. Review.
Cited by
-
Critical Role for AMPK in Metabolic Disease-Induced Chronic Kidney Disease.Int J Mol Sci. 2020 Oct 27;21(21):7994. doi: 10.3390/ijms21217994. Int J Mol Sci. 2020. PMID: 33121167 Free PMC article. Review.
-
NOX4-dependent regulation of ENaC in hypertension and diabetic kidney disease.FASEB J. 2020 Oct;34(10):13396-13408. doi: 10.1096/fj.202000966RR. Epub 2020 Aug 16. FASEB J. 2020. PMID: 32799394 Free PMC article.
-
TRPC Channels in the Physiology and Pathophysiology of the Renal Tubular System: What Do We Know?Int J Mol Sci. 2022 Dec 22;24(1):181. doi: 10.3390/ijms24010181. Int J Mol Sci. 2022. PMID: 36613622 Free PMC article. Review.
-
Nedd4-2 ablation in kidney improves glycaemic control in diabetic mice.Cell Death Dis. 2025 Jul 5;16(1):496. doi: 10.1038/s41419-025-07826-3. Cell Death Dis. 2025. PMID: 40617804 Free PMC article.
-
The key mediator of diabetic kidney disease: Potassium channel dysfunction.Genes Dis. 2023 Sep 22;11(4):101119. doi: 10.1016/j.gendis.2023.101119. eCollection 2024 Jul. Genes Dis. 2023. PMID: 38523672 Free PMC article. Review.
References
-
- Andersen H, Friis UG, Hansen PB, Svenningsen P, Henriksen JE, & Jensen BL (2015). Diabetic nephropathy is associated with increased urine excretion of proteases plasmin, prostasin and urokinase and activation of amiloride-sensitive current in collecting duct cells. Nephrol Dial Transplant, 30(5), 781–789. 10.1093/ndt/gfu402 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

