Nitro-Fatty Acid Logistics: Formation, Biodistribution, Signaling, and Pharmacology
- PMID: 31196614
- PMCID: PMC7121905
- DOI: 10.1016/j.tem.2019.04.009
Nitro-Fatty Acid Logistics: Formation, Biodistribution, Signaling, and Pharmacology
Abstract
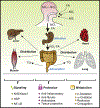
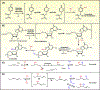
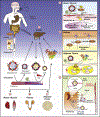
In addition to supporting cellular energetic demands and providing building blocks for lipid synthesis, fatty acids (FAs) are precursors of potent signaling molecules. In particular, the presence of conjugated double bonds on the fatty-acyl chain provides a preferential target for nitration generating nitro-FAs (NO2-FAs). The formation of NO2-FAs is a nonenzymatic process that requires reactive nitrogen species and occurs locally at the site of inflammation or during gastric acidification. NO2-FAs are electrophilic and display pleiotropic signaling actions through reversible protein alkylation. This review focuses on the endogenously formed NO2-FAs' mechanism of absorption, systemic distribution, signaling, and preclinical models. Understanding the dynamics of these processes will facilitate targeted dietary interventions and further the current pharmacological development aimed at low-grade inflammatory diseases.
Keywords: endogenous formation; inflammation; nitro-fatty acid; signaling; systemic distribution.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
Nitro-fatty acids: electrophilic signaling molecules in plant physiology.Planta. 2021 Nov 13;254(6):120. doi: 10.1007/s00425-021-03777-z. Planta. 2021. PMID: 34773515 Free PMC article. Review.
-
Nitro Fatty Acids (NO2-FAs): An Emerging Class of Bioactive Fatty Acids.Molecules. 2021 Dec 13;26(24):7536. doi: 10.3390/molecules26247536. Molecules. 2021. PMID: 34946618 Free PMC article. Review.
-
Nitro-fatty acid formation and metabolism.Nitric Oxide. 2018 Sep 1;79:38-44. doi: 10.1016/j.niox.2018.07.003. Epub 2018 Jul 10. Nitric Oxide. 2018. PMID: 30006146 Free PMC article. Review.
-
Electrophilic fatty acid nitroalkenes are systemically transported and distributed upon esterification to complex lipids.J Lipid Res. 2019 Feb;60(2):388-399. doi: 10.1194/jlr.M088815. Epub 2018 Dec 13. J Lipid Res. 2019. PMID: 30545956 Free PMC article.
-
Nitro-fatty acid metabolome: saturation, desaturation, beta-oxidation, and protein adduction.J Biol Chem. 2009 Jan 16;284(3):1461-73. doi: 10.1074/jbc.M802298200. Epub 2008 Nov 17. J Biol Chem. 2009. PMID: 19015269 Free PMC article.
Cited by
-
Targeting metabolism in aortic aneurysm and dissection: from basic research to clinical applications.Int J Biol Sci. 2023 Jul 31;19(12):3869-3891. doi: 10.7150/ijbs.85467. eCollection 2023. Int J Biol Sci. 2023. PMID: 37564200 Free PMC article. Review.
-
Nitro-fatty acids: electrophilic signaling molecules in plant physiology.Planta. 2021 Nov 13;254(6):120. doi: 10.1007/s00425-021-03777-z. Planta. 2021. PMID: 34773515 Free PMC article. Review.
-
A FABP4-PPARγ signaling axis regulates human monocyte responses to electrophilic fatty acid nitroalkenes.Redox Biol. 2020 Jan;29:101376. doi: 10.1016/j.redox.2019.101376. Epub 2019 Nov 10. Redox Biol. 2020. PMID: 31926616 Free PMC article.
-
Nitro-Oleic Acid-Mediated Nitroalkylation Modulates the Antioxidant Function of Cytosolic Peroxiredoxin Tsa1 during Heat Stress in Saccharomyces cerevisiae.Antioxidants (Basel). 2022 May 14;11(5):972. doi: 10.3390/antiox11050972. Antioxidants (Basel). 2022. PMID: 35624836 Free PMC article.
-
Electrophilic characteristics and aqueous behavior of fatty acid nitroalkenes.Redox Biol. 2021 Jan;38:101756. doi: 10.1016/j.redox.2020.101756. Epub 2020 Oct 12. Redox Biol. 2021. PMID: 33181478 Free PMC article.
References
-
- Burr GO and Burr MM (1973) Nutrition classics from The Journal of Biological Chemistry 82:345–67, 1929. A new deficiency disease produced by the rigid exclusion of fat from the diet. Nutr. Rev. 31, 248–249 - PubMed
-
- Holman RT (1998) The slow discovery of the importance of omega 3 essential fatty acids in human health. J. Nutr 128, 427S–433S - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

