Subcortical Substrates of Explore-Exploit Decisions in Primates
- PMID: 31196672
- PMCID: PMC6687547
- DOI: 10.1016/j.neuron.2019.05.017
Subcortical Substrates of Explore-Exploit Decisions in Primates
Abstract
The explore-exploit dilemma refers to the challenge of deciding when to forego immediate rewards and explore new opportunities that could lead to greater rewards in the future. While motivational neural circuits facilitate learning based on past choices and outcomes, it is unclear whether they also support computations relevant for deciding when to explore. We recorded neural activity in the amygdala and ventral striatum of rhesus macaques as they solved a task that required them to balance novelty-driven exploration with exploitation of what they had already learned. Using a partially observable Markov decision process (POMDP) model to quantify explore-exploit trade-offs, we identified that the ventral striatum and amygdala differ in how they represent the immediate value of exploitative choices and the future value of exploratory choices. These findings show that subcortical motivational circuits are important in guiding explore-exploit decisions.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of Interests
The authors declare no competing interests.
Figures



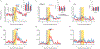
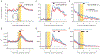
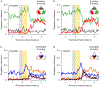
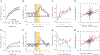
Comment in
-
Re-exploring Mechanisms of Exploration.Neuron. 2019 Aug 7;103(3):360-363. doi: 10.1016/j.neuron.2019.07.021. Neuron. 2019. PMID: 31394060
References
-
- Apicella P (2017). The role of the intrinsic cholinergic system of the striatum: What have we learned from TAN recordings in behaving animals? Neuroscience 360, 81–94. - PubMed
-
- Aston-Jones G, and Cohen JD (2005). An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci 28, 403–450. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

