Absence of complement component 3 does not prevent classical pathway-mediated hemolysis
- PMID: 31196848
- PMCID: PMC6595256
- DOI: 10.1182/bloodadvances.2019031591
Absence of complement component 3 does not prevent classical pathway-mediated hemolysis
Abstract
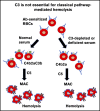
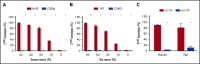
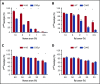
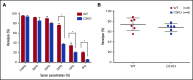
Complement component 3 (C3) is emerging as a potential therapeutic target. We studied complement-mediated hemolysis using normal and C3-depleted human sera, wild-type (WT) and C3-deficient rat sera, and WT and C3 knockout rat models. In all of the in vitro and in vivo experiments, we found that the loss of C3 did not prevent classical pathway-mediated hemolysis, but it did almost abolish alternative pathway-mediated hemolysis. Experiments using preassembled classical pathway C3 convertases confirmed that C4b2a directly activated complement component 5 (C5), leading to membrane attack complex formation and hemolysis. Our results suggest that targeting C3 should effectively inhibit hemolysis and tissue damage mediated by the alternative pathway of complement activation, but this approach might have limited efficacy in treating classical pathway-mediated pathological conditions.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures
References
-
- Molina H, Miwa T, Zhou L, et al. Complement-mediated clearance of erythrocytes: mechanism and delineation of the regulatory roles of Crry and DAF. Decay-accelerating factor. Blood. 2002;100(13):4544-4549. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

