Evidence for vesicle-mediated antigen export by the human pathogen Babesia microti
- PMID: 31196872
- PMCID: PMC6572159
- DOI: 10.26508/lsa.201900382
Evidence for vesicle-mediated antigen export by the human pathogen Babesia microti
Abstract
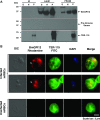
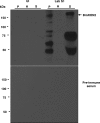
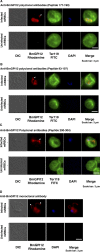
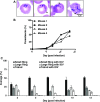
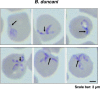
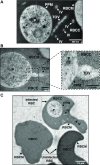
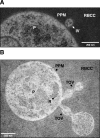
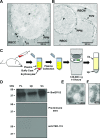
The apicomplexan parasite Babesia microti is the primary agent of human babesiosis, a malaria-like illness and potentially fatal tick-borne disease. Unlike its close relatives, the agents of human malaria, B. microti develops within human and mouse red blood cells in the absence of a parasitophorous vacuole, and its secreted antigens lack trafficking motifs found in malarial secreted antigens. Here, we show that after invasion of erythrocytes, B. microti undergoes a major morphogenic change during which it produces an interlacement of vesicles (IOV); the IOV system extends from the plasma membrane of the parasite into the cytoplasm of the host erythrocyte. We developed antibodies against two immunodominant antigens of the parasite and used them in cell fractionation studies and fluorescence and immunoelectron microscopy analyses to monitor the mode of secretion of B. microti antigens. These analyses demonstrate that the IOV system serves as a major export mechanism for important antigens of B. microti and represents a novel mechanism for delivery of parasite effectors into the host by this apicomplexan parasite.
© 2019 Thekkiniath et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
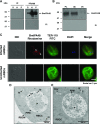
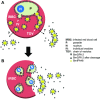
References
-
- Abraham A, Brasov I, Thekkiniath J, Kilian N, Lawres L, Gao R, DeBus K, He L, Yu X, Zhu G, et al. (2018) Establishment of a continuous in vitro culture of Babesia duncani in human erythrocytes reveals unusually high tolerance to recommended therapies. J Biol Chem 293: 19974–19981. 10.1074/jbc.ac118.005771 - DOI - PMC - PubMed
-
- Baranyai T, Herczeg K, Onodi Z, Voszka I, Modos K, Marton N, Nagy G, Mager I, Wood MJ, El Andaloussi S, et al. (2015) Isolation of exosomes from blood plasma: Qualitative and quantitative comparison of ultracentrifugation and size exclusion chromatography methods. PLoS One 10: e0145686 10.1371/journal.pone.0145686 - DOI - PMC - PubMed
-
- Ben Mamoun C, Allred DR (2018) Babesiosis In eLS. Chichester: John Wiley & Sons, Ltd.
-
- Boddey JA, O’Neill MT, Lopaticki S, Carvalho TG, Hodder AN, Nebl T, Wawra S, van West P, Ebrahimzadeh Z, Richard D, et al. (2016) Export of malaria proteins requires co-translational processing of the PEXEL motif independent of phosphatidylinositol-3-phosphate binding. Nat Commun 7: 10470 10.1038/ncomms10470 - DOI - PMC - PubMed
-
- Chen TK, Ben Mamoun C, Krause PJ (2015) Human babesiosis In Clinical Infectious Disease. Schlossberg D. (ed) Cambridge: Cambridge University Press; pp 1295–1301.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
