Treg-mediated prolonged survival of skin allografts without immunosuppression
- PMID: 31196957
- PMCID: PMC6613183
- DOI: 10.1073/pnas.1903165116
Treg-mediated prolonged survival of skin allografts without immunosuppression
Erratum in
-
Correction for Pilat et al., Treg-mediated prolonged survival of skin allografts without immunosuppression.Proc Natl Acad Sci U S A. 2020 May 5;117(18):10097. doi: 10.1073/pnas.2005821117. Proc Natl Acad Sci U S A. 2020. PMID: 32366648 Free PMC article. No abstract available.
Abstract
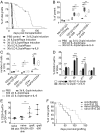
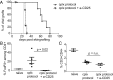
Injection of Interleukin-2 (IL-2) complexed with a particular anti-IL-2 monoclonal antibody (mab) JES6-1 has been shown to selectively expand CD4+Foxp3+ T regulatory T cells (Tregs) in vivo. Although the potency of this approach with regard to transplantation has already been proven in an islet transplantation model, skin graft survival could not be prolonged. Since the latter is relevant to human allograft survival, we sought to improve the efficiency of IL-2 complex (cplx) treatment for skin allograft survival in a stringent murine skin graft model. Here, we show that combining low doses of IL-2 cplxs with rapamycin and blockade of the inflammatory cytokine IL-6 leads to long-term (>75 d) survival of major histocompatibility complex-different skin allografts without the need for immunosuppression. Allograft survival was critically dependent on CD25+FoxP3+ Tregs and was not accompanied by impaired responsiveness toward donor alloantigens in vitro after IL-2 cplx treatment was stopped. Furthermore, second donor-type skin grafts were rejected and provoked rejection of the primary graft, suggesting that operational tolerance is not systemic but restricted to the graft. These findings plus the lack of donor-specific antibody formation imply that prolonged graft survival was largely a reflection of immunological ignorance. The results may represent a potentially clinically translatable strategy for the development of protocols for tolerance induction.
Keywords: interleukin-2; regulatory T cells; tolerance; transplantation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

