Region-specific and activity-dependent regulation of SVZ neurogenesis and recovery after stroke
- PMID: 31196958
- PMCID: PMC6612913
- DOI: 10.1073/pnas.1811825116
Region-specific and activity-dependent regulation of SVZ neurogenesis and recovery after stroke
Abstract
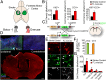
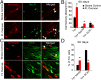
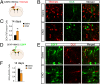
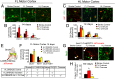
Stroke is the leading cause of adult disability. Neurogenesis after stroke is associated with repair; however, the mechanisms regulating poststroke neurogenesis and its functional effect remain unclear. Here, we investigate multiple mechanistic routes of induced neurogenesis in the poststroke brain, using both a forelimb overuse manipulation that models a clinical neurorehabilitation paradigm, as well as local manipulation of cellular activity in the peri-infarct cortex. Increased activity in the forelimb peri-infarct cortex via either modulation drives increased subventricular zone (SVZ) progenitor proliferation, migration, and neuronal maturation in peri-infarct cortex. This effect is sensitive to competition from neighboring brain regions. By using orthogonal tract tracing and rabies virus approaches in transgenic SVZ-lineage-tracing mice, SVZ-derived neurons synaptically integrate into the peri-infarct cortex; these effects are enhanced with forelimb overuse. Synaptic transmission from these newborn SVZ-derived neurons is critical for spontaneous recovery after stroke, as tetanus neurotoxin silencing specifically of the SVZ-derived neurons disrupts the formation of these synaptic connections and hinders functional recovery after stroke. SVZ-derived neurogenesis after stroke is activity-dependent, region-specific, and sensitive to modulation, and the synaptic connections formed by these newborn cells are functionally critical for poststroke recovery.
Keywords: astrocyte; motor; neurorehabilitation; plasticity; synapse.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Ohira K., et al. , Ischemia-induced neurogenesis of neocortical layer 1 progenitor cells. Nat. Neurosci. 13, 173–179 (2010). - PubMed
-
- Donega V., Raineteau O., Postnatal neural stem cells: Probing their competence for cortical repair. Neuroscientist 23, 605–615 (2017). - PubMed
-
- Arvidsson A., Collin T., Kirik D., Kokaia Z., Lindvall O., Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 8, 963–970 (2002). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

