Accurately cleavable goat β-lactoglobulin signal peptide efficiently guided translation of a recombinant human plasminogen activator in transgenic rabbit mammary gland
- PMID: 31196965
- PMCID: PMC6597847
- DOI: 10.1042/BSR20190596
Accurately cleavable goat β-lactoglobulin signal peptide efficiently guided translation of a recombinant human plasminogen activator in transgenic rabbit mammary gland
Abstract
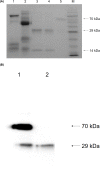
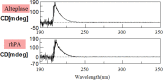
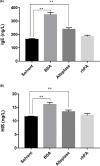
Poor expression is the key factor hampering the large-scale application of transgenic animal mammary gland bioreactors. A very different approach would be to evaluate the secretion of recombinant proteins into milk in response to a cleavable signal peptide of highly secreted lactoproteins.We previously reported rabbits harboring mammary gland-specific expression vector containing a fusion cDNA (goat β-lactoglobulin (BLG) signal peptide and recombinant human plasminogen activator (rhPA) coding sequences) expressed rhPA in the milk, but we did not realize the signal peptide contributed to the high rhPA concentration and did not mention it at that time. And the molecular structure and biological characteristics still remain unknown. So, rhPA in the milk was purified and characterized in the present study.rhPA was purified from the milk, and the purity of the recovered product was 98% with no loss of biological activity. Analysis of the N-terminal sequence, C-terminal sequence, and the molecular mass of purified rhPA revealed that they matched the theoretical design requirements. The active systemic anaphylaxis (ASA) reactions of the purified rhPA were negative. Taken together, these results indicated that the goat BLG signal peptide can efficiently mediate rhPA secretion into milk and was accurately cleaved off from rhPA by endogenous rabbit signal peptidase.We have reinforced the importance of a rhPA coding region fused to a cleavable heterologous signal peptide from highly secreted goat BLG to improve recombinant protein expression. It is anticipated that these findings will be widely applied to high-yield production of medically important recombinant proteins.
Keywords: BLG; mammary gland bioreactor; purification; rabbit; rhPA; signal peptide.
© 2019 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures
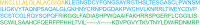

Similar articles
-
A novel recombinant human plasminogen activator: Efficient expression and hereditary stability in transgenic goats and in vitro thrombolytic bioactivity in the milk of transgenic goats.PLoS One. 2018 Aug 17;13(8):e0201788. doi: 10.1371/journal.pone.0201788. eCollection 2018. PLoS One. 2018. PMID: 30118482 Free PMC article.
-
High-level expression of a novel recombinant human plasminogen activator (rhPA) in the milk of transgenic rabbits and its thrombolytic bioactivity in vitro.Mol Biol Rep. 2016 Aug;43(8):775-83. doi: 10.1007/s11033-016-4020-0. Epub 2016 May 26. Mol Biol Rep. 2016. PMID: 27230577
-
Efficient increase of the novel recombinant human plasminogen activator expression level and stability through the use of homozygote transgenic rabbits.Int J Mol Med. 2018 Oct;42(4):2269-2275. doi: 10.3892/ijmm.2018.3754. Epub 2018 Jul 4. Int J Mol Med. 2018. PMID: 30015826
-
The mammary gland as a bioreactor: expression, processing, and production of recombinant proteins.J Mammary Gland Biol Neoplasia. 1998 Jul;3(3):337-50. doi: 10.1023/a:1018723712996. J Mammary Gland Biol Neoplasia. 1998. PMID: 10819519 Review.
-
Transgenic animal bioreactors.Transgenic Res. 2000;9(4-5):305-20. doi: 10.1023/a:1008934912555. Transgenic Res. 2000. PMID: 11131009 Free PMC article. Review.
Cited by
-
Evaluation of the α-casein (CSN1S1) locus as a potential target for a site-specific transgene integration.Sci Rep. 2022 May 14;12(1):7983. doi: 10.1038/s41598-022-12071-1. Sci Rep. 2022. PMID: 35568783 Free PMC article.
-
Computational design, expression, and characterization of a Plasmodium falciparum multi-epitope, multi-stage vaccine candidate (PfCTMAG).Heliyon. 2025 Jan 18;11(2):e42014. doi: 10.1016/j.heliyon.2025.e42014. eCollection 2025 Jan 30. Heliyon. 2025. PMID: 39906795 Free PMC article.
-
Development of innovative multi-epitope mRNA vaccine against central nervous system tuberculosis using in silico approaches.PLoS One. 2024 Sep 6;19(9):e0307877. doi: 10.1371/journal.pone.0307877. eCollection 2024. PLoS One. 2024. PMID: 39240891 Free PMC article.
-
Design of multi-epitope vaccine candidate against Brucella type IV secretion system (T4SS).PLoS One. 2023 Aug 10;18(8):e0286358. doi: 10.1371/journal.pone.0286358. eCollection 2023. PLoS One. 2023. PMID: 37561685 Free PMC article.
-
In silico designed novel multi-epitope mRNA vaccines against Brucella by targeting extracellular protein BtuB and LptD.Sci Rep. 2024 Mar 27;14(1):7278. doi: 10.1038/s41598-024-57793-6. Sci Rep. 2024. PMID: 38538674 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

