Protein Kinase C Downregulation Enhanced Extracellular Ca2+-Induced Relaxation of Isolated Mesenteric Arteries from Aged Dahl Salt-Sensitive Rats
- PMID: 31197021
- PMCID: PMC6697777
- DOI: 10.1124/jpet.119.258475
Protein Kinase C Downregulation Enhanced Extracellular Ca2+-Induced Relaxation of Isolated Mesenteric Arteries from Aged Dahl Salt-Sensitive Rats
Abstract
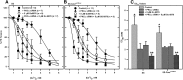
The Ca2+-sensing receptor (CaSR) detects small changes in extracellular calcium (Ca2+ e) concentration ([Ca2+]e) and transduces the signal into modulation of various signaling pathways. Ca2+-induced relaxation of isolated phenylephrine-contracted mesenteric arteries is mediated by the CaSR of the perivascular nerve. Elucidation of the regulatory mechanisms involved in vascular CaSR signaling may provide insights into the physiologic functions of the receptor and identify targets for the development of new treatments for cardiovascular pathologies such as hypertension. Protein kinase Cα (PKCα) is a critical regulator of multiple signaling pathways and can phosphorylate the CaSR leading to receptor desensitization. In this study, we used automated wire myography to investigate the effects of CaSR mutation and small-interfering RNA downregulation of PKCα on CaSR-mediated relaxation of phenylephrine-contracted mesenteric arteries from aged Dahl salt-sensitive (SS) rats on a low-salt diet. The data showed minimal relaxation responses of arteries to Ca2+ e in wild-type (SS) and CaSR mutant (SS-Casrem1Mcwi) rats. Mutation of the CaSR gene had no significant effect on relaxation. PKCα expression was similar in wild-type and mutant rats, and small-interfering RNA downregulation of PKCα and/or inhibition of PKC with the Ca2+-sensitive Gӧ 6976 resulted in a >80% increase in relaxation. Significant differences in EC50 values were observed between treated and untreated controls (P < 0.05 analysis of variance). The results indicate that PKCα plays an important role in the regulation of CaSR-mediated relaxation of mesenteric arteries, and its downregulation or pharmacological inhibition may lead to an increased Ca2+ sensitivity of the receptor and reversal of age-related changes in vascular tone. SIGNIFICANCE STATEMENT: G protein-coupled CaSR signaling leads to the regulation of vascular tone and may, therefore, play a vital role in blood pressure regulation. The receptor has several PKC phosphorylation sites in the C-terminal intracellular tail that mediate desensitization. We have previously shown that activation of the CaSR in neuronal cells leads to PKC phosphorylation, indicating that protein kinase C is an important regulator of CaSR function. Therefore, PKC in the CaSR signaling pathway in mesenteric arteries is a potential target for the development of new therapeutic approaches to treat hypertension and age-related vascular dysfunction. The present studies show that small-interfering RNA downregulation of PKCα and pharmacological inhibition of PKC enhanced CaSR-mediated relaxation of phenylephrine-contracted mesenteric arteries from aged Dahl salt-sensitive rats.
Copyright © 2019 by The American Society for Pharmacology and Experimental Therapeutics.
Figures
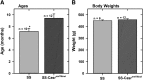
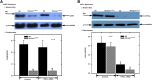

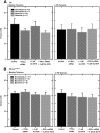
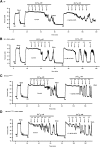
Similar articles
-
High Salt Upregulates Ca2+-Sensing Receptor Expression and Ca2+-Induced Relaxation of Contracted Mesenteric Arteries from Dahl Salt-Sensitive Rats.J Pharmacol Exp Ther. 2022 May;381(2):120-128. doi: 10.1124/jpet.121.001034. Epub 2022 Mar 19. J Pharmacol Exp Ther. 2022. PMID: 35306475 Free PMC article.
-
Nitric-oxide synthase knockout modulates Ca²⁺-sensing receptor expression and signaling in mouse mesenteric arteries.J Pharmacol Exp Ther. 2013 Jul;346(1):38-47. doi: 10.1124/jpet.113.205534. Epub 2013 May 2. J Pharmacol Exp Ther. 2013. PMID: 23639802 Free PMC article.
-
Mesenteric artery contraction and relaxation studies using automated wire myography.J Vis Exp. 2011 Sep 22;(55):3119. doi: 10.3791/3119. J Vis Exp. 2011. PMID: 21969063 Free PMC article.
-
Important roles of the Ca2+-sensing receptor in vascular health and disease.Life Sci. 2018 Sep 15;209:217-227. doi: 10.1016/j.lfs.2018.08.016. Epub 2018 Aug 8. Life Sci. 2018. PMID: 30098342 Review.
-
Role of the calcium-sensing receptor in regulating vascular function.J Cell Commun Signal. 2025 Feb 5;19(1):e70004. doi: 10.1002/ccs3.70004. eCollection 2025 Mar. J Cell Commun Signal. 2025. PMID: 39912052 Free PMC article. Review.
Cited by
-
Differential Proteomics Analysis of the Subcutaneous Connective Tissues in Alcian Blue Tracks along Conception Vessel and Adjacent Nonmeridian in Rats.Evid Based Complement Alternat Med. 2021 May 4;2021:5550694. doi: 10.1155/2021/5550694. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 34035822 Free PMC article.
-
Proteomics-based evaluation of the mechanism underlying vascular injury via DNA interstrand crosslinks, glutathione perturbation, mitogen-activated protein kinase, and Wnt and ErbB signaling pathways induced by crotonaldehyde.Clin Proteomics. 2022 Aug 24;19(1):33. doi: 10.1186/s12014-022-09369-7. Clin Proteomics. 2022. PMID: 36002804 Free PMC article.
-
Characterization of Contractile Machinery of Vascular Smooth Muscles in Hypertension.Life (Basel). 2021 Jul 16;11(7):702. doi: 10.3390/life11070702. Life (Basel). 2021. PMID: 34357074 Free PMC article. Review.
-
The Influences of Perinatal Androgenic Exposure on Cardiovascular and Metabolic Disease of Offspring of PCOS.Reprod Sci. 2023 Nov;30(11):3179-3189. doi: 10.1007/s43032-023-01286-w. Epub 2023 Jun 28. Reprod Sci. 2023. PMID: 37380913 Review.
-
High Salt Upregulates Ca2+-Sensing Receptor Expression and Ca2+-Induced Relaxation of Contracted Mesenteric Arteries from Dahl Salt-Sensitive Rats.J Pharmacol Exp Ther. 2022 May;381(2):120-128. doi: 10.1124/jpet.121.001034. Epub 2022 Mar 19. J Pharmacol Exp Ther. 2022. PMID: 35306475 Free PMC article.
References
-
- Angus JA, Wright CE. (2000) Techniques to study the pharmacodynamics of isolated large and small blood vessels. J Pharmacol Toxicol Methods 44:395–407. - PubMed
-
- Awumey EM, Howlett AC, Putney JW, Jr, Diz DI, Bukoski RD. (2007) Ca(2+) mobilization through dorsal root ganglion Ca(2+)-sensing receptor stably expressed in HEK293 cells. Am J Physiol Cell Physiol 292:C1895–C1905. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

