The heme-sensitive regulator SbnI has a bifunctional role in staphyloferrin B production by Staphylococcus aureus
- PMID: 31197035
- PMCID: PMC6663872
- DOI: 10.1074/jbc.RA119.007757
The heme-sensitive regulator SbnI has a bifunctional role in staphyloferrin B production by Staphylococcus aureus
Abstract
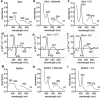
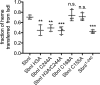
Staphylococcus aureus infection relies on iron acquisition from its host. S. aureus takes up iron through heme uptake by the iron-responsive surface determinant (Isd) system and by the production of iron-scavenging siderophores. Staphyloferrin B (SB) is a siderophore produced by the 9-gene sbn gene cluster for SB biosynthesis and efflux. Recently, the ninth gene product, SbnI, was determined to be a free l-serine kinase that produces O-phospho-l-serine (OPS), a substrate for SB biosynthesis. Previous studies have also characterized SbnI as a DNA-binding regulatory protein that senses heme to control sbn gene expression for SB synthesis. Here, we present crystal structures at 1.9-2.1 Å resolution of a SbnI homolog from Staphylococcus pseudintermedius (SpSbnI) in both apo form and in complex with ADP, a product of the kinase reaction; the latter confirmed the active-site location. The structures revealed that SpSbnI forms a dimer through C-terminal domain swapping and a dimer of dimers through intermolecular disulfide formation. Heme binding had only a modest effect on SbnI enzymatic activity, suggesting that its two functions are independent and structurally distinct. We identified a heme-binding site and observed catalytic heme transfer between a heme-degrading protein of the Isd system, IsdI, and SbnI. These findings support the notion that SbnI has a bifunctional role contributing precursor OPS to SB synthesis and directly sensing heme to control expression of the sbn locus. We propose that heme transfer from IsdI to SbnI enables S. aureus to control iron source preference according to the sources available in the environment.
Keywords: crystal structure; heme; iron metabolism; kinetics; siderophore.
© 2019 Verstraete et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Figures
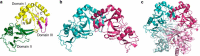

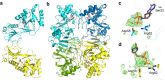

Similar articles
-
SbnI is a free serine kinase that generates O -phospho-l-serine for staphyloferrin B biosynthesis in Staphylococcus aureus.J Biol Chem. 2018 Apr 20;293(16):6147-6160. doi: 10.1074/jbc.RA118.001875. Epub 2018 Feb 26. J Biol Chem. 2018. PMID: 29483190 Free PMC article.
-
A Heme-responsive Regulator Controls Synthesis of Staphyloferrin B in Staphylococcus aureus.J Biol Chem. 2016 Jan 1;291(1):29-40. doi: 10.1074/jbc.M115.696625. Epub 2015 Nov 3. J Biol Chem. 2016. PMID: 26534960 Free PMC article.
-
Staphylococcus aureus heme and siderophore-iron acquisition pathways.Biometals. 2019 Jun;32(3):409-424. doi: 10.1007/s10534-019-00188-2. Epub 2019 Mar 25. Biometals. 2019. PMID: 30911924
-
Structural biology of heme binding in the Staphylococcus aureus Isd system.J Inorg Biochem. 2010 Mar;104(3):341-8. doi: 10.1016/j.jinorgbio.2009.09.012. Epub 2009 Sep 26. J Inorg Biochem. 2010. PMID: 19853304 Review.
-
Iron-regulated surface determinants (Isd) of Staphylococcus aureus: stealing iron from heme.Microbes Infect. 2004 Apr;6(4):390-7. doi: 10.1016/j.micinf.2003.12.008. Microbes Infect. 2004. PMID: 15101396 Review.
Cited by
-
The Role of Iron in Staphylococcus aureus Infection and Human Disease: A Metal Tug of War at the Host-Microbe Interface.Front Cell Dev Biol. 2022 Mar 24;10:857237. doi: 10.3389/fcell.2022.857237. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35399529 Free PMC article. Review.
-
Linking Virulence and Iron Limitation Response in Staphylococcus aureus: The sRNA IsrR Is Involved in SaeRS Activation.J Proteome Res. 2025 Jul 4;24(7):3324-3342. doi: 10.1021/acs.jproteome.5c00059. Epub 2025 Jun 2. J Proteome Res. 2025. PMID: 40456523 Free PMC article.
-
Elucidation of Gram-Positive Bacterial Iron(III) Reduction for Kaolinite Clay Refinement.Molecules. 2021 May 21;26(11):3084. doi: 10.3390/molecules26113084. Molecules. 2021. PMID: 34064160 Free PMC article.
-
Iron Metabolism at the Interface between Host and Pathogen: From Nutritional Immunity to Antibacterial Development.Int J Mol Sci. 2020 Mar 20;21(6):2145. doi: 10.3390/ijms21062145. Int J Mol Sci. 2020. PMID: 32245010 Free PMC article. Review.
-
Phenotypic and genotypic assessment of iron acquisition in diverse bovine-associated non-aureus staphylococcal strains.Vet Res. 2024 Jan 12;55(1):6. doi: 10.1186/s13567-023-01260-z. Vet Res. 2024. PMID: 38217046 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

