Bicarbonate is essential for protein-tyrosine phosphatase 1B (PTP1B) oxidation and cellular signaling through EGF-triggered phosphorylation cascades
- PMID: 31197039
- PMCID: PMC6699834
- DOI: 10.1074/jbc.RA119.009001
Bicarbonate is essential for protein-tyrosine phosphatase 1B (PTP1B) oxidation and cellular signaling through EGF-triggered phosphorylation cascades
Abstract
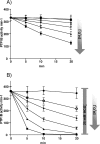
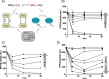
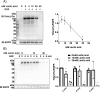
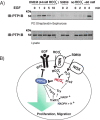
Protein-tyrosine phosphatases (PTPs) counteract protein tyrosine phosphorylation and cooperate with receptor-tyrosine kinases in the regulation of cell signaling. PTPs need to undergo oxidative inhibition for activation of cellular cascades of protein-tyrosine kinase phosphorylation following growth factor stimulation. It has remained enigmatic how such oxidation can occur in the presence of potent cellular reducing systems. Here, using in vitro biochemical assays with purified, recombinant protein, along with experiments in the adenocarcinoma cell line A431, we discovered that bicarbonate, which reacts with H2O2 to form the more reactive peroxymonocarbonate, potently facilitates H2O2-mediated PTP1B inactivation in the presence of thioredoxin reductase 1 (TrxR1), thioredoxin 1 (Trx1), and peroxiredoxin 2 (Prx2) together with NADPH. The cellular experiments revealed that intracellular bicarbonate proportionally dictates total protein phosphotyrosine levels obtained after stimulation with epidermal growth factor (EGF) and that bicarbonate levels directly correlate with the extent of PTP1B oxidation. In fact, EGF-induced cellular oxidation of PTP1B was completely dependent on the presence of bicarbonate. These results provide a plausible mechanism for PTP inactivation during cell signaling and explain long-standing observations that growth factor responses and protein phosphorylation cascades are intimately linked to the cellular acid-base balance.
Keywords: bicarbonate; epidermal growth factor receptor (EGFR); oxidative inactivation; peroxiredoxin; peroxymonocarbonate; phosphatase; phosphorylation cascade; protein-tyrosine phosphatase (PTP); redox regulation; redox signaling; thioredoxin reductase.
© 2019 Dagnell et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
-
- Andersen J. N., Mortensen O. H., Peters G. H., Drake P. G., Iversen L. F., Olsen O. H., Jansen P. G., Andersen H. S., Tonks N. K., and Møller N. P. (2001) Structural and evolutionary relationships among protein tyrosine phosphatase domains. Mol. Cell. Biol. 21, 7117–7136 10.1128/MCB.21.21.7117-7136.2001 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

