Cellular Effects of Butyrate on Vascular Smooth Muscle Cells are Mediated through Disparate Actions on Dual Targets, Histone Deacetylase (HDAC) Activity and PI3K/Akt Signaling Network
- PMID: 31197106
- PMCID: PMC6628026
- DOI: 10.3390/ijms20122902
Cellular Effects of Butyrate on Vascular Smooth Muscle Cells are Mediated through Disparate Actions on Dual Targets, Histone Deacetylase (HDAC) Activity and PI3K/Akt Signaling Network
Abstract
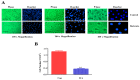
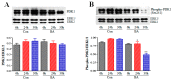
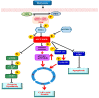
Vascular remodeling is a characteristic feature of cardiovascular diseases. Altered cellular processes of vascular smooth muscle cells (VSMCs) is a crucial component in vascular remodeling. Histone deacetylase inhibitor (HDACI), butyrate, arrests VSMC proliferation and promotes cell growth. The objective of the study is to determine the mechanism of butyrate-induced VSMC growth. Using proliferating VSMCs exposed to 5 mM butyrate, immunoblotting studies are performed to determine whether PI3K/Akt pathway that regulates different cellular effects is a target of butyrate-induced VSMC growth. Butyrate inhibits phosphorylation-dependent activation of PI3K, PDK1, and Akt, eliciting differential effects on downstream targets of Akt. Along with previously reported Ser9 phosphorylation-mediated GSK3 inactivation leading to stability, increased expression and accumulation of cyclin D1, and epigenetic histone modifications, inactivation of Akt by butyrate results in: transcriptional activation of FOXO1 and FOXO3 promoting G1 arrest through p21Cip1/Waf1 and p15INK4B upregulation; inactivation of mTOR inhibiting activation of its targets p70S6K and 4E-BP1 impeding protein synthesis; inhibition of caspase 3 cleavage and downregulation of PARP preventing apoptosis. Our findings imply butyrate abrogates Akt activation, causing differential effects on Akt targets promoting convergence of cross-talk between their complimentary actions leading to VSMC growth by arresting proliferation and inhibiting apoptosis through its effect on dual targets, HDAC activity and PI3K/Akt pathway network.
Keywords: Akt; butyrate; histone deacetylase inhibitor; signaling; vascular smooth muscle cells.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Angiotensin II-induced histone deacetylase 5 phosphorylation, nuclear export, and Egr-1 expression are mediated by Akt pathway in A10 vascular smooth muscle cells.Am J Physiol Heart Circ Physiol. 2021 Apr 1;320(4):H1543-H1554. doi: 10.1152/ajpheart.00683.2020. Epub 2021 Feb 19. Am J Physiol Heart Circ Physiol. 2021. PMID: 33606583
-
Butyrate, an HDAC inhibitor, stimulates interplay between different posttranslational modifications of histone H3 and differently alters G1-specific cell cycle proteins in vascular smooth muscle cells.Biomed Pharmacother. 2010 Dec;64(10):733-40. doi: 10.1016/j.biopha.2010.09.017. Biomed Pharmacother. 2010. PMID: 20970954 Free PMC article.
-
The short-chain fatty acid butyrate accelerates vascular calcification via regulation of histone deacetylases and NF-κB signaling.Vascul Pharmacol. 2022 Oct;146:107096. doi: 10.1016/j.vph.2022.107096. Epub 2022 Aug 8. Vascul Pharmacol. 2022. PMID: 35952961
-
Inhibition of histone deacetylase activity by butyrate.J Nutr. 2003 Jul;133(7 Suppl):2485S-2493S. doi: 10.1093/jn/133.7.2485S. J Nutr. 2003. PMID: 12840228 Review.
-
Insulin's actions on vascular tissues: Physiological effects and pathophysiological contributions to vascular complications of diabetes.Mol Metab. 2021 Oct;52:101236. doi: 10.1016/j.molmet.2021.101236. Epub 2021 Apr 18. Mol Metab. 2021. PMID: 33878400 Free PMC article. Review.
Cited by
-
Implication of Gut Microbiota in Cardiovascular Diseases.Oxid Med Cell Longev. 2020 Sep 26;2020:5394096. doi: 10.1155/2020/5394096. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 33062141 Free PMC article. Review.
-
Phenotypic Modulation of Macrophages and Vascular Smooth Muscle Cells in Atherosclerosis-Nitro-Redox Interconnections.Antioxidants (Basel). 2021 Mar 26;10(4):516. doi: 10.3390/antiox10040516. Antioxidants (Basel). 2021. PMID: 33810295 Free PMC article. Review.
-
Histone deacetylases: Regulation of vascular homeostasis via endothelial cells and vascular smooth muscle cells and the role in vascular pathogenesis.Genes Dis. 2024 Jan 22;11(6):101216. doi: 10.1016/j.gendis.2024.101216. eCollection 2024 Nov. Genes Dis. 2024. PMID: 39281836 Free PMC article. Review.
-
Ferulic Acid Alleviates Atherosclerotic Plaques by Inhibiting VSMC Proliferation Through the NO/p21 Signaling pathway.J Cardiovasc Transl Res. 2022 Aug;15(4):865-875. doi: 10.1007/s12265-021-10196-8. Epub 2022 Jan 6. J Cardiovasc Transl Res. 2022. PMID: 34993756 Free PMC article.
-
Time-Dependent Changes in Effects of Butyrate on Human Gingival Fibroblasts.Clin Exp Dent Res. 2025 Jun;11(3):e70120. doi: 10.1002/cre2.70120. Clin Exp Dent Res. 2025. PMID: 40275488 Free PMC article.
References
-
- Go A.S., Muzaffarabad D., Roger V.L., Benjamin E.J., Berry J.D., Borden W.B., Bravata D.M., Dai S., Ford E.S., Fox C.S., et al. Heart disease and stroke statistics–2013 update: A report from the American Heart Association. Circulation. 2013;127:143–152. doi: 10.1161/CIR.0b013e318282ab8f. - DOI - PubMed
-
- Ranganna K., Yatsu F.M., Mathew O.P. Insights into the pathogenesis and intervention of atherosclerosis. Vasc. Dis. Prev. 2006;3:375–390. doi: 10.2174/1567270000603010052. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

