Vimentin activation in early apoptotic cancer cells errands survival pathways during DNA damage inducer CPT treatment in colon carcinoma model
- PMID: 31197132
- PMCID: PMC6565729
- DOI: 10.1038/s41419-019-1690-2
Vimentin activation in early apoptotic cancer cells errands survival pathways during DNA damage inducer CPT treatment in colon carcinoma model
Abstract
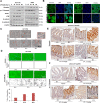
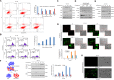
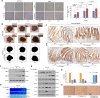
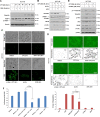
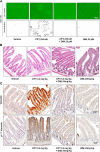
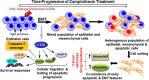
Epithelial to mesenchymal transitions (EMT) is a preparatory process for cancer cells to attain motility and further metastasis to distant sites. Majority of DNA damaging drugs have shown to develop EMT as one of the major mechanisms to attain drug resistance. Here we sought to understand the resistance/survival instincts of cancer cells during initial phase of drug treatment. We provide a tangible evidence of stimulation of EMT factors in Apc knockout colorectal carcinoma model. Our results implied that CPT-treated Apc knockout cohorts depicted increased pro-invasive and pro-survival factors (Vimentin/pser38Vimentin & NFκB). Moreover, by cell sorting experiment, we have observed the expression of Vimentin in early apoptotic cells (AnnexinV positive) from 36 to 48 h of CPT treatment. We also observed the expression of chimeric Sec-AnnexinV-mvenus protein in migrated cells on transwell membrane recapitulating signatures of early apoptosis. Notably, induction of Vimentin-mediated signaling (by CPT) delayed apoptosis progression in cells conferring survival responses by modulating the promoter activity of NFκB. Furthermore, our results unveiled a novel link between Vimentin and ATM signaling, orchestrated via binding interaction between Vimentin and ATM kinase. Finally, we observed a significant alteration of crypt-villus morphology upon combination of DIM (EMT inhibitor) with CPT nullified the background EMT signals thus improving the efficacy of the DNA damaging agent. Thus, our findings revealed a resistance strategy of cancer cells within a very initial period of drug treatment by activating EMT program, which hinders the cancer cells to achieve later phases of apoptosis thus increasing the chances of early migration.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Interleukin 6-triggered ataxia-telangiectasia mutated kinase activation facilitates epithelial-to-mesenchymal transition in lung cancer by upregulating vimentin expression.Exp Cell Res. 2019 Aug 15;381(2):165-171. doi: 10.1016/j.yexcr.2019.05.011. Epub 2019 May 14. Exp Cell Res. 2019. PMID: 31100307
-
Consumption of high-fat diet induces tumor progression and epithelial-mesenchymal transition of colorectal cancer in a mouse xenograft model.J Nutr Biochem. 2012 Oct;23(10):1302-13. doi: 10.1016/j.jnutbio.2011.07.011. Epub 2012 Jan 4. J Nutr Biochem. 2012. PMID: 22221675
-
miR-22 and miR-214 targeting BCL9L inhibit proliferation, metastasis, and epithelial-mesenchymal transition by down-regulating Wnt signaling in colon cancer.FASEB J. 2019 Apr;33(4):5411-5424. doi: 10.1096/fj.201801798RR. Epub 2019 Jan 30. FASEB J. 2019. PMID: 30698996
-
Vimentin in cancer and its potential as a molecular target for cancer therapy.Cell Mol Life Sci. 2011 Sep;68(18):3033-46. doi: 10.1007/s00018-011-0735-1. Epub 2011 Jun 3. Cell Mol Life Sci. 2011. PMID: 21637948 Free PMC article. Review.
-
Targeting vimentin: a multifaceted approach to combatting cancer metastasis and drug resistance.Cancer Metastasis Rev. 2024 Mar;43(1):363-377. doi: 10.1007/s10555-023-10154-7. Epub 2023 Nov 28. Cancer Metastasis Rev. 2024. PMID: 38012357 Review.
Cited by
-
LncRNA PTENP1/miR-21/PTEN Axis Modulates EMT and Drug Resistance in Cancer: Dynamic Boolean Modeling for Cell Fates in DNA Damage Response.Int J Mol Sci. 2024 Jul 29;25(15):8264. doi: 10.3390/ijms25158264. Int J Mol Sci. 2024. PMID: 39125832 Free PMC article.
-
Loss of EGF receptor polarity enables homeostatic imbalance in epithelial-cell models.Mol Biol Cell. 2023 Nov 1;34(12):ar116. doi: 10.1091/mbc.E23-04-0133. Epub 2023 Aug 30. Mol Biol Cell. 2023. PMID: 37647145 Free PMC article.
-
Oroxylin A: A Promising Flavonoid for Prevention and Treatment of Chronic Diseases.Biomolecules. 2022 Aug 26;12(9):1185. doi: 10.3390/biom12091185. Biomolecules. 2022. PMID: 36139025 Free PMC article. Review.
-
Comparative Study of Organoids from Patient-Derived Normal and Tumor Colon and Rectal Tissue.Cancers (Basel). 2020 Aug 15;12(8):2302. doi: 10.3390/cancers12082302. Cancers (Basel). 2020. PMID: 32824266 Free PMC article.
-
Vimentin-mediated buffering of internal integrin β1 pool increases survival of cells from anoikis.BMC Biol. 2024 Jun 24;22(1):139. doi: 10.1186/s12915-024-01942-w. BMC Biol. 2024. PMID: 38915055 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

