Environmental pH and peptide signaling control virulence of Streptococcus pyogenes via a quorum-sensing pathway
- PMID: 31197146
- PMCID: PMC6565748
- DOI: 10.1038/s41467-019-10556-8
Environmental pH and peptide signaling control virulence of Streptococcus pyogenes via a quorum-sensing pathway
Abstract
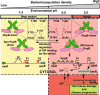
Bacteria control gene expression in concert with their population density by a process called quorum sensing, which is modulated by bacterial chemical signals and environmental factors. In the human pathogen Streptococcus pyogenes, production of secreted virulence factor SpeB is controlled by a quorum-sensing pathway and environmental pH. The quorum-sensing pathway consists of a secreted leaderless peptide signal (SIP), and its cognate receptor RopB. Here, we report that the SIP quorum-sensing pathway has a pH-sensing mechanism operative through a pH-sensitive histidine switch located at the base of the SIP-binding pocket of RopB. Environmental acidification induces protonation of His144 and reorganization of hydrogen bonding networks in RopB, which facilitates SIP recognition. The convergence of two disparate signals in the SIP signaling pathway results in induction of SpeB production and increased bacterial virulence. Our findings provide a model for investigating analogous crosstalk in other microorganisms.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

